- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events
Electron- and Photo-Induced Processes
2-Dimensional Charge Delocalization Aids Charge Separation in Molecular Systems
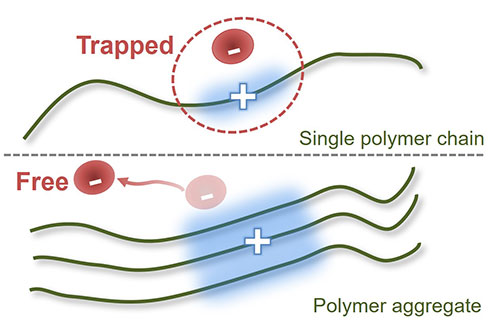
The electron acceptor F4TCNQ p-dopes aggregates “nanowires” of poly(3-hexylthiophene) in nonpolar solvents but does not dope unaggregated chains. The standard free energy change for the charge transfer to form an ion pair is ΔG°et = -0.21 eV. The dissociation constant to produce free ions in toluene by DC conductivity is K°d = 1 × 10-8 ± 50% (ΔG°d = 0.48 ± 0.05 eV). This remarkably large K°d, for ions in such a low polarity medium, may reflect interchain delocalization of the hole. The particular characteristics of this material system enables determination of both ΔG°et and ΔG°d, to find the overall free energy change from the two neutral species to completely separated ions in nonpolar media. It is endergonic by +0.27 ± 0.05 eV in contrast to -0.6 eV estimated from reported HOMO LUMO differences, illustrating the challenges that persist in determining such energetics. Steady state microwave conductivity experiments on doped aggregates confirm that holes in the aggregates cannot easily escape their dopant counterion, but at higher dopant concentrations, holes become mobile. These results provide insight into the mechanisms of charge separation involving intermolecularly delocalized charges in nonpolar media, an integral process in organic photovoltaic devices and doped molecular films.
Adv. Mat., 2019, 31, 1806863 DOI: 10.1002/adma.201806863




