- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Surface Electrochemistry and Electrocatalysis
Increasing stability and activity by alloying cores
Stability is one of the main requirements for operating electrocatalysts. Platinum is the best-known catalyst for oxygen reduction in cathodes, but it undergoes dissolution during potential changes while driving electric vehicles. We have shown for several systems that alloying cores with small amounts of Au, or modifying surfaces with Au clusters, improved stability and activity of such catalysts. Notable examples include the stability and activity of the PtML/Pd/C electrocatalyst upon alloying Pd with a small amount of Au to obtain PdAu alloy cores. In situ X-ray absorption spectroscopy (XAS) clearly demonstrated considerable retardation of Pd oxidation for Pd9Au alloy nanoparticles with increasing potentials when compared with Pd nanoparticles. DFT calculations for the Pd9Au1@Pt/C core-shell nanoparticles indicate that Au atoms segregate preferentially at defect sites in PtML; if Au atoms mend the defective sites, Pd dissolution from the cores will be effectively suppressed. The Au dopants may also enable the addition of non-noble constituents while retaining stability. The catalytic benefits of a Pd substrate can be enhanced by adding Ni, Fe or Co due to electronic and size effects. In addition, they make cheaper cores.
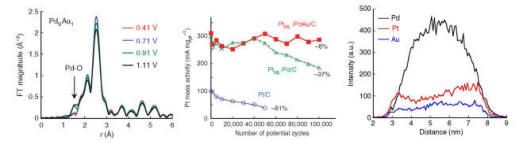
Alloy-induced performance stability of PtML catalysts: Delayed Pd
oxidation in PdAu alloy (left panel); Stability over 100,000 potential cycles (center);
components’ distribution after test
Nature communications | 3:1115 | DOI: 10.1038/ncomms2124 |




