- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Surface Electrochemistry and Electrocatalysis
Tetrahedral Pd Nanocrystals
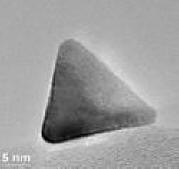
High - and low -magnification TEM image of the PdTH/C
We have shown that slightly contracted Pt(111) facets are among the most active surfaces for the ORR. That was motivation to synthesize tetrahedral Pd (PdTH) nanocrystals with cleaned surfaces. Considering the specific requirements of the ORR, their facet-specific electrochemical properties make PdTH a new interesting support for PtML catalysts.
The surfaces of the PdTH core are composed of (111) facets wherein the Pd atoms are highly coordinated and have low surface energy. Our results revealed that in comparison with spherical Pd/C-supported PtML or pure Pt, the PdTH-supported PtML features more surface contraction and a downshift of the d-band relative to the Fermi level. The PtML/PdTH produced a specific- and mass-activity of 0.64 mA/cm2 Pt, and 1.02 A/mgPt, which are higher than that of the Pt NPs consisting of (110)- and (111)-facets. These geometric- and electronic-effects determine the higher activity of PtML/PdTH/C for the ORR compared to that of PtML/Pd/C. This shape-property interdependence opens up a new approach in research on Pt-based ORR electrocatalysts that may be important for future catalyst development.
Z. Phys. Chem. 226 (2012) 1025–1038




