- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Surface Electrochemistry and Electrocatalysis
Making Ir capable of C-C bond splitting in ethanol oxidation
We previously demonstrated the unique ability of PtRhSnO2 catalyst to oxidize ethanol to CO2 at low potentials. More recently, we have shown that the PtIr/SnO2/C catalyst with high Ir content has outstanding catalytic properties with the most negative EOR onset potential and reasonably good selectivity toward ethanol complete oxidation to CO2, higher than those of Pt/C and Pt/SnO2/C.
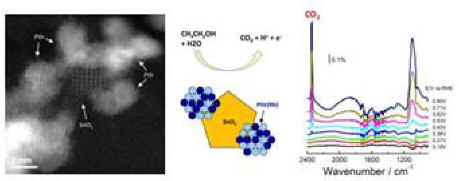
Structure, synthesis and IR spectra in ETOH oxidation on IrPtSnO2/C
Among the systems studied, PtIr/SnO2/C catalyst with highest Ir content, that is, the catalyst with atomic ratio Pt:Ir:Sn = 1:1:1, showed the most negative EOR onset potential and considerably improved capability for C−C bond splitting. PtRh/SnO2/C electrocatalysts with a suitable Rh content, that is, catalysts with atomic ratio Pt:Rh:Sn = 1:½:1 and 1:⅓:1, exhibit the highest selectivity toward ethanol total oxidation. The highest CO2 production efficiency of the above catalysts is observed with the PtRh/SnO2 (with atomic ratio Pt/Rh/Sn of 1:½:1 and 1:⅓:1) > PtIrRh/SnO2 (with atomic ratio Pt:Ir:Rh:Sn of 1:1:1:1). This finding provides new information on the C−C bond splitting electrocatalysis and can help in designing new catalysts with enhanced activity and selectivity in ethanol electrooxidation.
Journal of the American Chemical Society 135, 132 (Jan, 2013).




