- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Surface Electrochemistry and Electrocatalysis
Clusters: The ultimate low metal content electrocatalysts
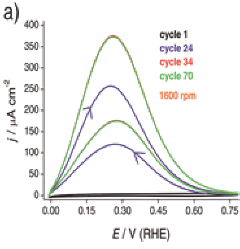
Oxidation of H2 on Au clusters as a function of cluster size growing with cycling
The ultimate reduction in usage of noble metal in catalysts can be achieved by using metal clusters, defined as nanoparticles smaller than ~ 100 atoms. Their properties differ from those of the bulk or bigger nanoparticles since they lose their metallic nature with decreasing size and behave more like a semiconductor. The existence of band gaps in their electronic structure together with geometrical arrangements confers novel properties. We have shown the first example of catalytic activity of Au clusters, prepared by a new surfactantless method for the hydrogen oxidation reaction (HOR) in 0.1 M HClO4. We term these molecular-like species Au atomic quantum clusters (AuAQCs) to emphasize the observed quantum effects on their catalytic behavior. Their HOR activity is up 180-fold higher than that of bulk Au depending on the surface orientation of Au. Initially, small Au clusters of circa 2 - 5 atoms cannot catalyze the HOR. They were activated by potential cycling in the range –10 mV to 790 mV (vs. RHE).
Since experimental conditions do not allow direct or indirect determination of the size of Au clusters after their activation, we developed a model based on electronic structure of Au clusters which predicts a volcano-type dependence of activity vs. cluster size and that only Au clusters which contain 10-50 atoms are expected to be HOR active. This relationship stems from the requirement that the position of the conduction band (CB) of the Au cluster is below the standard redox potential for the HOR, while the valence band (VB) is below the H 1s-Au d antibonding resonance, so preventing repulsion between the clusters and H2. The consideration of the HOR data and our model suggests that AuAQCs are 20-30 atoms. The proposed model can also be used to estimate the minimal Pt and Pd cluster size required for acquiring the activity for HOR which makes it a general tool for making such estimates.
Angew. Chem. I.E. submitted.




