Magnesium Protects Tantalum, a Promising Material for Making Qubits
Thin-film coating prevents oxidation that impairs superconductivity and quantum coherence
February 5, 2024
 enlarge
enlarge
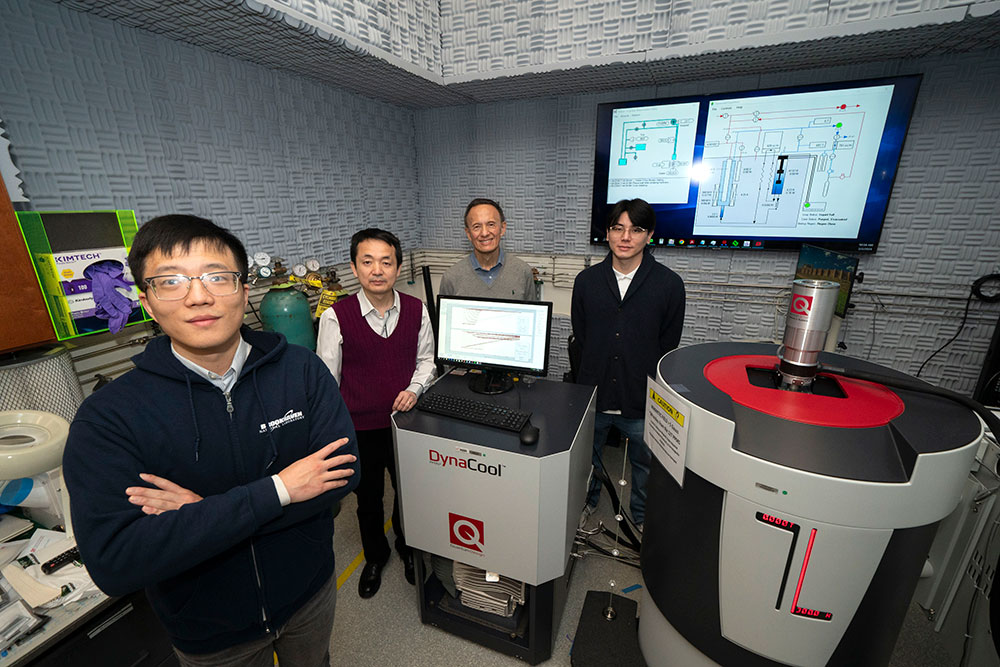
Chenyu Zhou, a research associate in the Center for Functional Nanomaterials (CFN) at Brookhaven National Laboratory and first author on the study, with Mingzhao Liu (CFN), Yimei Zhu (CMPMS), and Junsik Mun (CFN and CMPMSD), at the DynaCool Physical Property Measurement System (PPMS) in CFN. The team used this tool to make tantalum thin films with and without a protective magnesium layer so they could determine whether the magnesium coating would minimize tantalum oxidation. (Jessica Rotkiewicz/Brookhaven National Laboratory)
UPTON, NY—Scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory have discovered that adding a layer of magnesium improves the properties of tantalum, a superconducting material that shows great promise for building qubits, the basis of quantum computers. As described in a paper just published in the journal Advanced Materials, a thin layer of magnesium keeps tantalum from oxidizing, improves its purity, and raises the temperature at which it operates as a superconductor. All three may increase tantalum’s ability to hold onto quantum information in qubits.
This work builds on earlier studies in which a team from Brookhaven’s Center for Functional Nanomaterials (CFN), Brookhaven’s National Synchrotron Light Source II (NSLS-II), and Princeton University sought to understand the tantalizing characteristics of tantalum, and then worked with scientists in Brookhaven’s Condensed Matter Physics & Materials Science (CMPMS) Department and theorists at DOE’s Pacific Northwest National Laboratory (PNNL) to reveal details about how the material oxidizes.
Those studies showed why oxidation is an issue.
 enlarge
enlarge
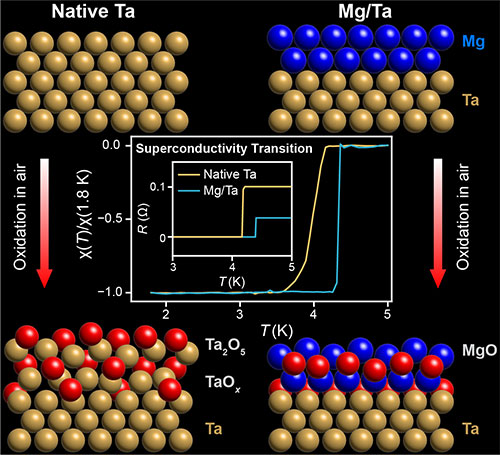
These molecular diagrams compare the oxidation of native tantalum (Ta), left, in which the oxide penetrates the Ta lattice, with that of tantalum coated with an ultrathin layer of magnesium (Mg), right. Mg acts as an oxygen barrier, effectively suppressing Ta oxidation, and pulls impurities from Ta. Both improve the superconducting properties of the underlaying Ta thin film—shown in the graphs as a sharper transition to superconductivity at a higher temperature. (Brookhaven National Laboratory)
“When oxygen reacts with tantalum, it forms an amorphous insulating layer that saps tiny bits of energy from the current moving through the tantalum lattice. That energy loss disrupts quantum coherence—the material’s ability to hold onto quantum information in a coherent state,” explained CFN scientist Mingzhao Liu, a lead author on the earlier studies and the new work.
While the oxidation of tantalum is usually self-limiting—a key reason for its relatively long coherence time—the team wanted to explore strategies to further restrain oxidation to see if they could improve the material’s performance.
“The reason tantalum oxidizes is that you have to handle it in air and the oxygen in air will react with the surface,” Liu explained. “So, as chemists, can we do something to stop that process? One strategy is to find something to cover it up.”
All this work is being carried out as part of the Co-design Center for Quantum Advantage (C2QA), a Brookhaven-led national quantum information science research center. While ongoing studies explore different kinds of cover materials, the new paper describes a promising first approach: coating the tantalum with a thin layer of magnesium.
“When you make a tantalum film, it is always in a high-vacuum chamber, so there is not much oxygen to speak of,” said Liu. “The problem always happens when you take it out. So, we thought, without breaking the vacuum, after we put the tantalum layer down, maybe we can put another layer, like magnesium, on top to block the surface from interacting with the air.”
Studies using transmission electron microscopy to image structural and chemical properties of the material, atomic layer by atomic layer, showed that the strategy to coat tantalum with magnesium was remarkably successful. The magnesium formed a thin layer of magnesium oxide on the tantalum surface that appears to keep oxygen from getting through.
“Electron microscopy techniques developed at Brookhaven Lab enabled direct visualization not only of the chemical distribution and atomic arrangement within the thin magnesium coating layer and the tantalum film but also of the changes of their oxidation states,” said Yimei Zhu, a study co-author from CMPMS. “This information is extremely valuable in comprehending the material’s electronic behavior,” he noted.
X-ray photoelectron spectroscopy studies at NSLS-II revealed the impact of the magnesium coating on limiting the formation of tantalum oxide. The measurements indicated that an extremely thin layer of tantalum oxide—less than one nanometer thick—remains confined directly beneath the magnesium/tantalum interface without disrupting the rest of the tantalum lattice.
 enlarge
enlarge
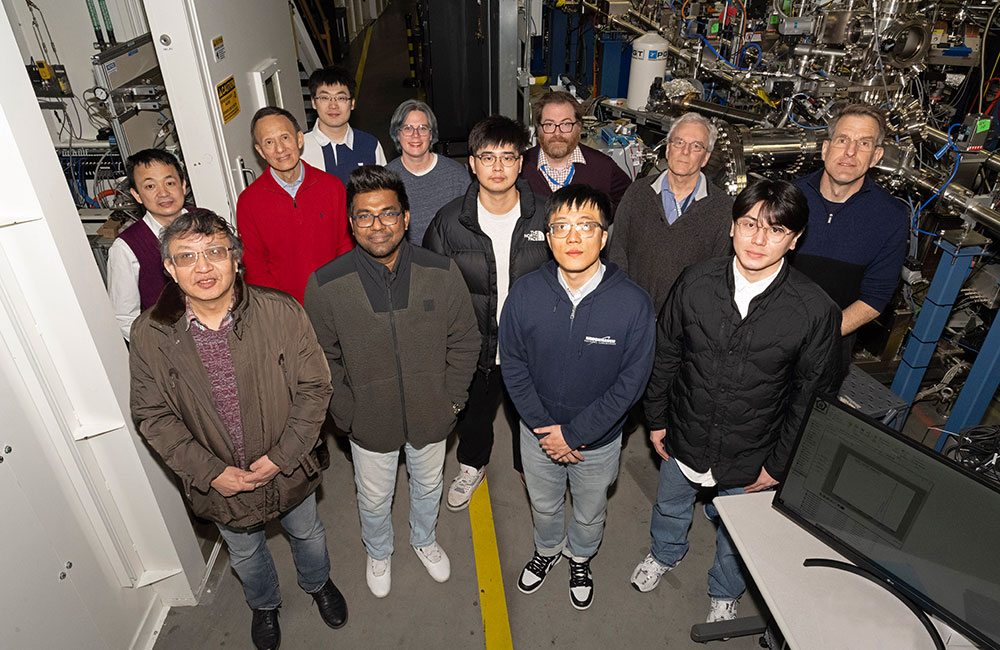
Members of the research team at the Spectroscopy Soft and Tender 2 (SST-2) beamline at the National Synchrotron Light Source II (NSLS-II). Front row (left to right): Xiao Tong (CFN), Aswin Kumar Anbalagan (NSLS II), Chenyu Zhou (CFN), Junsik Mun (CFN & CMPMS). Second row (left to right): Mingzhao Liu (CFN), Yimei Zhu (CMPMS), Juntao Yao (CMPMS), Steven Hulbert (NSLS II), Kim Kisslinger (CFN). Third row (left to right): Ruoshui Li (CFN), Ashley Head (CFN), Conan Weiland (NIST). (Kevin Coughlin/Brookhaven National Laboratory)
“This is in stark contrast to uncoated tantalum, where the tantalum oxide layer can be more than three nanometers thick—and significantly more disruptive to the electronic properties of tantalum,” said study co-author Andrew Walter, a lead beamline scientist in the Soft X-ray Scattering & Spectroscopy program at NSLS-II.
Collaborators at PNNL then used computational modeling at the atomic scale to identify the most likely arrangements and interactions of the atoms based on their binding energies and other characteristics. These simulations helped the team develop a mechanistic understanding of why magnesium works so well.
At the simplest level, the calculations revealed that magnesium has a higher affinity for oxygen than tantalum does.
“While oxygen has a high affinity to tantalum, it is ‘happier’ to stay with the magnesium than with the tantalum,” said Peter Sushko, one of the PNNL theorists. “So, the magnesium reacts with oxygen to form a protective magnesium oxide layer. You don’t even need that much magnesium to do the job. Just two nanometers of thickness of magnesium almost completely blocks the oxidation of tantalum.”
 enlarge
enlarge
Peter Sushko, a theorist at DOE's Pacific Northwest National Laboratory involved in the computational modeling that helped explain the properties of tantalum during oxidation both with and without a protective magnesium coating. (PNNL)
The scientists also demonstrated that the protection lasts a long time: “Even after one month, the tantalum is still in pretty good shape. Magnesium is a really good oxygen barrier,” Liu concluded.
The magnesium had an unexpected beneficial effect: It “sponged out” inadvertent impurities in the tantalum and, as a result, raised the temperature at which it operates as a superconductor.
“Even though we are making these materials in a vacuum, there is always some residual gas—oxygen, nitrogen, water vapor, hydrogen. And tantalum is very good at sucking up these impurities,” Liu explained. “No matter how careful you are, you will always have these impurities in your tantalum.”
But when the scientists added the magnesium coating, they discovered that its strong affinity for the impurities pulled them out. The resulting purer tantalum had a higher superconducting transition temperature.
That could be very important for applications because most superconductors must be kept very cold to operate. In these ultracold conditions, most of the conducting electrons pair up and move through the material with no resistance.
“Even a slight elevation in the transition temperature could reduce the number of remaining, unpaired electrons,” Liu said, potentially making the material a better superconductor and increasing its quantum coherence time.
“There will have to be follow-up studies to see if this material improves qubit performance,” Liu said. “But this work provides valuable insights and new materials design principles that could help pave the way to the realization of large-scale, high-performance quantum computing systems.”
This research was funded by the DOE Office of Science. The scientists used the Spectroscopy Soft and Tender Beamlines (SST-1 and SST-2) at NSLS-II, which are operated by the National Institute of Standards and Technology (NIST); Materials Synthesis & Characterization, Proximal Probe, and Electron Microscopy facilities at CFN; facilities of the Electron Microscopy and Nanostructure Group and Advanced Energy Materials Group in CMPMS; and computational resources of the National Energy Research Scientific Computing Center (NERSC) at DOE’s Lawrence Berkeley National Laboratory. CFN, NSLS-II, and NERSC are DOE Office of Science user facilities. The study included additional co-authors from CFN, CMPMS, NSLS-II, PNNL, Princeton University, Stony Brook University, and NIST.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on social media. Find us on Instagram, LinkedIn, X, and Facebook.
2024-21677 | INT/EXT | Newsroom