Understanding the Tantalizing Benefits of Tantalum for Qubits
August 30, 2023
 enlarge
enlarge
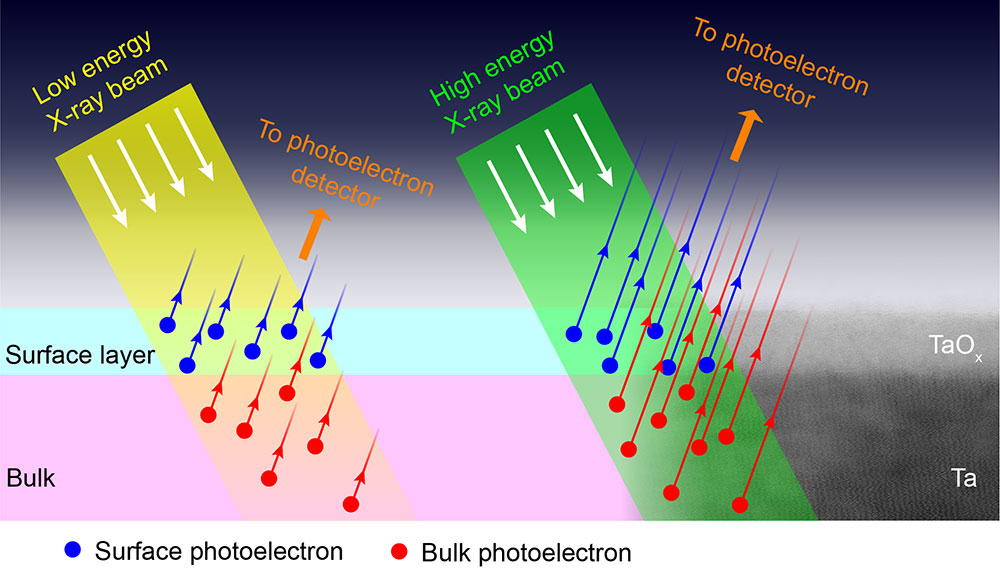
Depth profile at the surface of a superconducting qubit material (Ta), and its correlation with qubit decoherence using synchrotron spectroscopy, electron microscopy, and data analytics.
Scientific Achievement
CFN users collaborated with CFN staff on a study to correlate tantalum surface oxidation states with coherence losses in superconducting qubits. Developing a variable energy X-ray photoelectron spectroscopy methodology was key to revealing the depth profile of tantalum oxidation.
Significance and Impact
Tantalum is a promising material for superconducting qubits and has enabled record-long coherence times. Understanding the underlying reasons for qubit decoherence can yield material design pathways to ever better qubit performance.
Research Details
Over the past few decades, superconducting qubits have emerged as one of the leading hardware platforms for realizing a quantum processor. Consequently, researchers have made significant efforts to understand the loss channels that limit the coherence times of superconducting qubits. A major source of loss has been attributed to two-level systems that are present at the material interfaces. It is recently shown that replacing the metal in the capacitor of a transmon with tantalum yields record relaxation and coherence times for superconducting qubits, motivating a detailed study of the tantalum surface. In this work, the chemical profile of the surface of tantalum films grown on c-plane sapphire using variable energy x-ray photoelectron spectroscopy (VEXPS) is studied. The different oxidation states of tantalum that are present in the native oxide resulting from exposure to air are identified and their distribution through the depth of the film is measured. Furthermore, it is shown how the volume and depth distribution of these tantalum oxidation states can be altered by various chemical treatments. Correlating these measurements with detailed measurements of quantum devices may elucidate the underlying microscopic sources.
- Tantalum surface oxidation profiles were built using a unified data analysis tool and compared between surfaces processed with different chemical treatments.
- CFN Materials Synthesis and Electron Microscopy Facilities, along with the NSLS-Il/NIST SST-1 and SST-2 beamlines, were used for this study.
CFN Capabilities: The Materials Synthesis & Characterization Facility was used for Ta thin sample preparation and processing; the Electron Microscopy Facility was used to study the microscopic structures of the Ta film surfaces.
Publication Reference
McLellan R.A., Dutta A., Zhou C.Y., … Liu M. Z.,* Walter A.L.,* de Leon N.P.* “Chemical profiles of the oxides on tantalum in state of the art superconducting circuits.” Advanced Science 2023, 2300921.
* Corresponding authors
DOI: 10.1002/advs.202300921
https://doi.org/10.1002/advs.202300921
OSTI: www.osti.gov/biblio/1973289
Acknowledgment of Support
This material was based upon work primarily supported by the U.S. Department of Energy, Office of Science, National Quantum Information Science Research Centers, Co-design Center for Quantum Advantage (C2QA) under contract number DE-SC0012704. Film characterization and processing was partly supported by the National Science Foundation (RAISE DMR-1839199). This research used resources of the Spectroscopy Soft and Tender Beamlines (SST-1 and SST-2) of the National Synchrotron Light Source II and the Electron Microscopy and Materials Synthesis & Characterization facilities of the Center for Functional Nano-materials (CFN), U.S. Department of Energy Office of Science Facilities at Brookhaven National Laboratory under contract no. DE-SC0012704. The authors acknowledge the use of Princeton’s Imaging and Analysis Center (IAC), which is partially supported by the Princeton Center for Complex Materials (PCCM), the National Science Foundation (NSF) Materials Research Science and Engineering Center (MRSEC; DMR-2011750). Some chemical treatments were performed in the Princeton Institute for the Science and Technology of Materials (PRISM) cleanroom. This project was supported in part by the U.S. Department of Energy, Office of Science, Office of Workforce Development for Teachers and Scientists (WDTS) under the Science Undergraduate Laboratory Internships Program (SULI).
2023-21455 | INT/EXT | Newsroom









