Cause of Cathode Degradation Found for Nickel-rich Materials
To discover the cause of cathode degradation, scientists used multiple x-ray-based techniques and found a potential remedy
March 31, 2019
 enlarge
enlarge
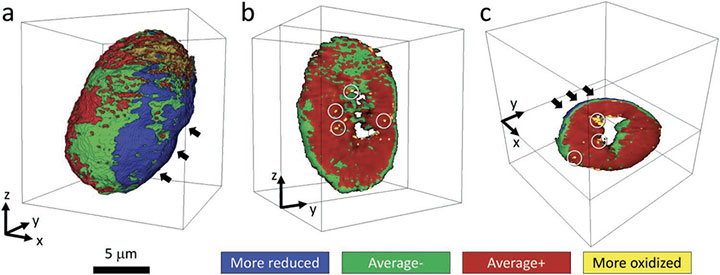
The three images show 3-D distributions of different chemical phases with different nickel (Ni) oxidation states within a Ni-rich secondary particle that was aggressively cycled. Scientists observed state-of-charge heterogeneity that led to performance degradation, which was reconstructed by a hybrid supervised-and-unsupervised machine learning algorithm that facilitates the extraction of the otherwise unavailable information.
The Science
Using machine learning techniques, scientists revealed and visualized chemical outliers that cause capacity fading upon high voltage charging in Nickel-rich-layered (Ni-rich-layered) cathodes.
The Impact
Ni-rich layered materials are promising candidates for high energy density lithium-ion (Li-ion) battery cathodes. This study offers a pathway to overcome the capacity fading problem.
Summary
Ni-rich-layered materials LiNi1-x-yMnxCoyO2 (NMC) are promising cathode candidates for high energy density lithium-ion battery systems. Unfortunately, they suffer from capacity fading upon cycling—in particular, when they are charged to high voltages, which are required to achieve the desired energy and power density.
A team of researchers tackled this problem by perform synchrotron-based x-ray spectroscopy, diffraction, and imaging experiments on the cathode materials that were subjected to high voltage cycling. For these measurements they used the Inner-Shell Spectroscopy (ISS) and X-ray Powder Diffraction (XPD) beamlines of the National Synchrotron Light Source II (NSLS-II)—a U.S. Department of Energy (DOE) Office of Science User Facility at DOE’s Brookhaven National Laboratory—and beamline 6-2C of the Stanford Synchrotron Radiation Light Source (SSRL)—another DOE Office of Science User Facility at SLAC National Accelerator Laboratory.
Although the bulk averaged experimental probes suggested the material is rather robust under aggressive charging to high voltages, the machine-learning-assisted nano-resolution spectro-microscopy facilitated the identification and visualization of undesired minority phases in the material. These minority phases are often overlooked because they are small in the total population and sparse in the spatial distribution. The machine learning methodology helped the scientists search through a large amount of synchrotron imaging data and identify the subset with “suspicious” spectroscopic fingerprints.
They concluded that, while the chemical heterogeneity is ubiquitous, the particle surface and the isolated domains in the particle undergo different side reactions and cause the performance degradation in different mechanisms. Further comparison of particles with different initial morphology suggests that the hollow particles are more robust to cracking than the solid particles. They pointed out that morphological engineering could be an effective way of controlling the mechanical disintegration of the particles.
Download the research summary slide
Related Links
Feature Story: “Cause of Cathode Degradation Identified for Nickel-rich Materials”
Contact
Xiao-Qing Yang
Brookhaven National Laboratory
xyang@bnl.gov
Enyuan Hu
Brookhaven National Laboratory
enhu@bnl.gov
Publications
Y. Mao, X. Wang, S. Xia, K. Zhang, C. Wei, S. Bak, Z. Shadike, X. Liu, Y. Yang, R. Xu, P. Pianetta, S. Ermon, E. Stavitski, K. Zhao, Z. Xu, F. Lin, X.-Q. Yang, E. Hu, Y. Liu. “High-Voltage Charging-Induced Strain, Heterogeneity, and Micro-Cracks in Secondary Particles of a Nickel-Rich Layered Cathode Material”, Adv. Funct. Mater. 1900247 (2019). DOI: 10.1002/adfm.201900247
Funding
The work done at Brookhaven National Laboratory was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Vehicle Technology Office of the U.S. Department of Energy, through the Advanced Battery Materials Research (BMR) Program, including Battery500 Consortium under Contract No. DE-SC0012704. This research used beamlines 8-ID and 28-ID-2 of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. Use of beamline 6-2 at the Stanford Synchrotron Radiation Light Source, SLAC National Accelerator Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The work was also supported by the National Science Foundation under Grant Nos. DMR-1832613 and DMR-1832707.
2019-16769 | INT/EXT | Newsroom









