Potential New Ion Conductor Based on Quantum Materials
Research team merges materials science and physics in new study
September 30, 2018
 enlarge
enlarge
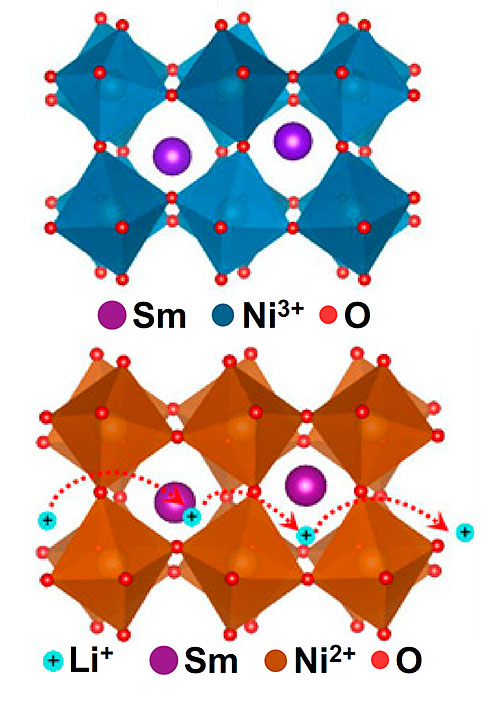
The top image shows the crystal structure of pristine SmNiO3 (SNO), which is a narrow-gapped semiconductor at room temperature. When lithiated (Li-SNO), the material becomes both an insulator and a lithium ion conductor, shown in the bottom image. Image credit: PNAS, 1805029115 (2018)
The Science
Scientists created a new solid-state ion conductor by incorporating lithium ions into rare-earth perovskite samarium nickelates.
The Impact
This ion-conductor has potential applications in electrochemical devices, nonvolatile memory, neuromorphic computing, and biomimicry.
Summary
In many systems, ranging from batteries to brains, the transport of ions plays a crucial role in transmitting information or signals. Currently, the leading ion-conducting materials are liquid and organic, but the development of solid and inorganic ion conductors could have broad applications in energy conversion, bioengineering, and information processing.
In traditional design approaches for new ion-conductors, scientists primarily try to dope a material, a process by which a different atom is introduced to the material in order to add new ions. However, this approach is limited by the amount, or concentration, of the added atoms to the material, which in turn limits ionic conductivity.
In this study, a team of scientists demonstrated a new class of lithium ion (Li-ion) conducting quantum materials in the perovskite family. These perovskite nickelates can be used as Li-ion shuttles with simultaneous suppression of electronic transport via a Mott transition. Adding lithium to these materials expands the structure in way that enables fast Li-ion conduction with reduced activation energy.
To fully understand the specific behavior of the electrons in the nickel and oxygen atoms inside the material, the researchers used a technique called x-ray absorption spectroscopy at the In situ and Operando Soft X-ray Spectroscopy (IOS) beamline at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility at DOE’s Brookhaven National Laboratory. The scientists combined these measurements with additional research methods and techniques to receive more in-depth knowledge about the material and its properties. They further presented a generalization of this approach with results on other rare-earth perovskite nickelates, as well as dopants such as sodium ions (Na+). The results highlight the potential of quantum materials and emergent physics in design of ion conductors.
Download the research summary slide
Related Links
Feature Story: Lithium Ions Flow Through Solid Material
Contact
Michele Kotiuga
Rutgers University
mkotiuga@physics.rutgers.edu
Karin M. Rabe
Rutgers University
kmrabe@physics.rutgers.edu
Publication
Y. Sun, M. Kotiuga, D. Lim, B. Narayanan, M. Cherukara, Z. Zhang, Y. Dong, R. Kou, C. Sun, Q. Lu, I. Waluyo, A. Hunt, H. Tanaka, A. N. Hattori, S. Gamage, Y. Abate, V. G. Pol, H. Zhou, S. K. R. S. Sankaranarayanan, B. Yildiz, K. M. Rabe, S. Ramanathan. Strongly correlated perovskite lithium ion shuttles. PNAS, 1805029115 (2018). DOI: 10.1073/pnas.1805029115
Funding
This work was supported by National Science Foundation Grants 1609898 and 1553251, Air Force Office of Scientific Research Grant FA9550-16-1-0159, Office of Naval Research grants N00014-17-1-2770 and N00014-18-1-2397, Army Research Office Grant W911NF-16-1-0042, Air Force Office of Scientific Research Grant FA9559-16-1-0172, Massachusetts Institute of Technology Materials Research Science and Engineering Center (MRSEC) through the MRSEC Program of the National Science Foundation under Award DMR-1419807, Japan Society for the Promotion of Science KAKENHI Grant 15KK0236. This research used resources of the Argonne Leadership Computing Facility, Center for Nanoscale Materials, and the Advanced Photon Source, which are supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract DE-AC02-06CH11357. This research used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the Office of Science of the US DOE under Contract DE-AC02-05CH11231. This research used resources 23-ID-2 beamline of the National Synchrotron Light Source II, a US DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract DE-SC0012704.
2018-17471 | INT/EXT | Newsroom