Multimodal Research Provides Insight into Lithium-Sulfur Batteries
Scientists gained insights into the chemical reactions and possible side effects of lithium-sulfur batteries using a combination of x-ray characterization techniques
January 31, 2018
 enlarge
enlarge
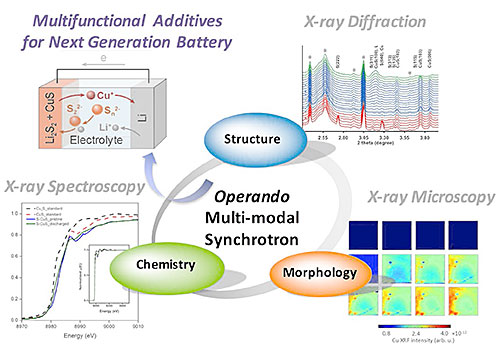
To fully understand the fundamental behavior and the reaction mechanisms of lithium-sulfur batteries with S/CuS hybrid electrodes, the scientists used three different synchrotron techniques to study the chemistry, structure, and morphology of the battery. Image credit: Sci. Rep. 7, 12976 (2017)
The Science
The detailed mechanism of CuS dissolution and its role in the electrochemical discharge of a Lithium-sulfur (Li-S) battery were uncovered using an in operando, multimodal approach.
The Impact
Li-S batteries are promising new electrochemical energy storage devices but their fundamental chemistry needs to be understood.
Summary
In this work, scientists successfully employed a multimodal approach to study a new type of lithium-sulfur battery and gave a new description of the battery’s chemistry. Lithium-sulfur (Li-S) batteries with conductive metal sulfides as multi-functional additives are promising new electrochemical energy storage devices, but their fundamental chemistry needs to be understood to ensure the additives that improve the batteries’ capacity don’t pose additional challenges such as parasitic chemical reactions.
Studying such complex reactions presents a challenge because the scientists need experimental methods that can track the chemical and structural evolution of the system during an electrochemical process.
Here, the scientists used various x-ray characterization techniques that offer complementary insights into the chemical reactions and possible side effects of these batteries. X-ray powder diffraction, x-ray fluorescence imaging, and x-ray absorption spectroscopy were used to resolve the different stages of the reaction during lithiation and de-lithiation of a Li-S battery with copper sulfides (CuS) as the multifunctional cathode additive. The scientists used the Submicron Resolution X-ray Spectroscopy (SRX) beamline, Inner-Shell Spectroscopy (ISS) beamline, and X-ray Powder Diffraction (XPD) beamline to perform all the different characterization measurements. All three beamlines are part of the materials characterization suite of the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory.
With these complementary techniques, the scientists identified crystalline phases, amorphous phases and the elemental distribution. They also described the dissolution mechanism: The lithiation of CuS starts at the very end of discharge to form amorphous Cu1+xS. During this process, CuS interacts strongly with soluble low-order polysulfide species. The dissolved Cu ions then migrate to the anode side and alter the anode, causing the fast capacity-fade.
Download the research summary slide
Related Links:
Feature Story: “Multi-Modal Operando X-Ray Study Yields New Insights on Lithium-Sulfur Batteries”
Contact
Yu-chen Karen Chen-Wiegart
Brookhaven National Laboratory/ Stony Brook University
ycchen@bnl.gov
Hong Gan
Brookhaven National Laboratory
hgan@bnl.gov
Publication
K. Sun, C. Zhao, C. Lin, E. Stavitski, G. Williams, J. Bai, E. Dooryhee, K. Attenkofer, J. Thieme, Y. K. Chen-Wiegart & H. Gan. Operando Multi-modal Synchrotron Investigation for Structural and Chemical Evolution of Cupric Sulfide (CuS) Additive in Li-S battery. Scientific Reports 7, 12976 (2017). DOI:10.1038/s41598-017-12738-0
Funding
This work was supported by the U.S. Department of Energy (DOE) Office of Energy Efficiency and Renewable Energy under the Advanced Battery Materials Research (BMR) program, Contract No. DE-SC0012704. This research used resources 5-ID (SRX), 8-ID (ISS) and 28-ID-2 (XPD) beamlines of the National Synchrotron Light Source II, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704.
2018-17474 | INT/EXT | Newsroom









