Simultaneous Ignition of CO Catalytic Oxidation
This new scientific discovery will benefit applications such as car exhaust cleaning and fuels cells
January 31, 2021
 enlarge
enlarge
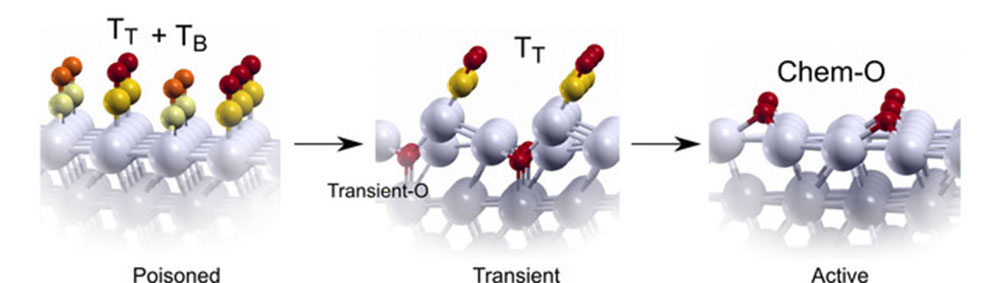
The three different stages of simultaneous ignition of catalytic CO oxidation: (from left to right) before the ignition, transient phase during ignition and active catalytic phase. Image credit: Fernando Garcia-Martinez, et. al. Angew. Chem. Int. Ed. 59 (45), 20037-20043 (2020).
The Science
Scientists discovered that the key to simultaneous ignition of the catalytic oxidation of carbon monoxide (CO), at different platinum (Pt) surfaces, is oxygen buildup below the surface.
The Impact
Catalytic oxidation of CO is highly relevant for many applications, such as car exhaust cleaning; this work offers new insights into the reaction mechanism.
Summary
From car exhaust cleaning to water gas shift reactions in fuels cells and industrial processing, catalytic oxidation of carbon monoxide (CO) by platinum-like metals, or (Pt)-group metals, has enormous technological, industrial, and environmental significance and has, therefore, been studied for the last four decades by many researchers. However, most research studies have investigated the reaction mechanism under high or ultra-high vacuum conditions, which leads to a lack of fundamental understanding in ambient pressure region.
In this work, a team of scientists has studied catalytic oxidation of CO on curved Pt surfaces under ambient pressure conditions to solve the following question: Why does the reaction always ignite simultaneously across all surfaces of the catalyst?
The team used a joint density functional theory and experimental approach to examine the reaction mechanism. For their experimental studies, the scientists used ambient pressure x-ray photoelectron spectroscopy (AP-XPS) at the In situ and Operando Soft X-ray Spectroscopy (IOS) Beamline at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory.
Through their analysis, the team found that the ignition of the reaction is triggered by a transient phase that is characterized by small amounts of oxygen mixed with the CO poisoning layer. This transient layer leads to almost equivalent CO chemisorption energy at terraces and steps, and hence to similar ignition conditions across the whole surface. The team also found that varying the reaction conditions and the annealing rates changes the ignition temperature and the composition of the active phase after ignition, but it does not alter the simultaneous ignition process at all Pt crystal planes.
Download the research summary slide
Contact
J. Enrique Ortega
Universidad del País Vasco
enrique.ortega@ehu.es
Publication
F. Garcia-Martinez, C. García-Fernández, J. P. Simonovis, A. Hunt, A. Walter, I. Waluyo, F. Bertram, L. R. Merte, M. Shipilin, S. Pfaff, S. Blomberg, J. Zetterberg, J. Gustafson, E. Lundgren, D. Sánchez-Portal, F. Schiller, J. E. Ortega Catalytic Oxidation of CO on a Curved Pt(111) Surface: Simultaneous Ignition at All Facets through a Transient CO-O Complex. Angewandte Chemie International Edition 59 (45), 20037-20043 (2020). DOI: 10.1002/anie.202007195
Funding
We acknowledge financial support from the Spanish Ministry of Science and Innovation (Grants MAT-2017-88374-P, PID2019-107338RB-C63, PID2019-107338RB-C66), the Basque Government (Grants IT-1255-19, IT-1246-19), the Knut and Alice Wallenberg foundation (DNR KAW 2015.0058 “Atomistic Design of new Catalysts”) and the Swedish Research Council (DNR 349-2011-6491 “Catalysis on the atomic scale”). This research used resources of the 23-ID-2 (IOS) beamline of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DESC0012704.
2021-18844 | INT/EXT | Newsroom