Two Catalysts Are Better Than One
Scientists fit two co-catalysts on one nanosheet for better water purification
March 31, 2020
 enlarge
enlarge
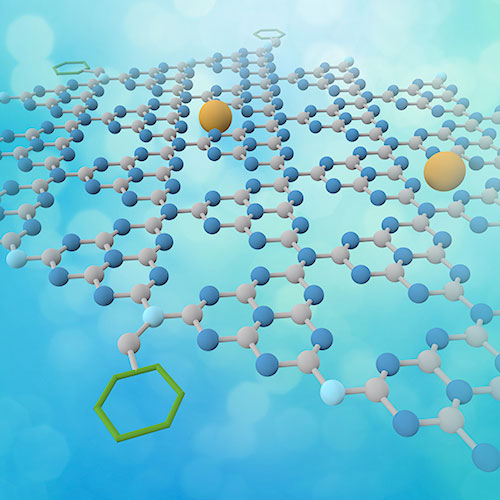
The image shows a 2D nanosheet with the following two co-catalysts in their respective places: a single-atom Co catalyst (yellow) sitting in the voids, and anthraquinone (green) attached to the edges of the sheet.Image credit: PNAS 117 (12) 6376-6382 (2020)
The Science
Scientists designed and demonstrated the efficiency a new catalytic material made of two spatially separated co-catalysts on a nanosheet for hydrogen peroxide synthesis.
The Impact
Using hydrogen peroxide for water purification is more environmentally friendly than many alternatives; however, the efficiency of the reaction is low. This new material provides a novel pathway to higher efficiency in future applications.
Summary
A team of scientists collaborated to design and test a new, two-dimensional (2D), catalytic material that can be used to improve water purification using hydrogen peroxide. While water treatment with hydrogen peroxide is environmentally friendly, the two-part chemical process that drives it is not very efficient. So far, scientists have struggled to improve the efficiency of the process through catalysis because each part of the reaction needs its own catalyst—called a co-catalyst—and the co-catalysts can’t be next to each other.
The trick of the newly designed catalytic material and its efficiency is that the scientists managed to place two co-catalysts – one for each part of the reaction – onto to different locations on a thin nanosheet. The beauty is in its simplicity; one of the co-catalysts, a single cobalt (Co) atom, sits in the center of the sheet, whereas the other one, a molecule called anthraquinone, is placed around the edges. This would not be possible with catalysts made of nanomaterials, since they would be ‘too big’ for this purpose.
After the scientists synthesized the new two-in-one catalytic material, they needed to figure out if the co-catalysts would stay separated during an actual reaction and how well this new 2D catalyst would perform. The scientists started their investigation at the hard x-ray Inner Shell Spectroscopy (ISS) beamline at the National Synchrotron Light Source II (NSLS-II) using a technique called x-ray absorption spectroscopy. NSLS-II is a Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory. This technique helped the team to learn more about the local structure of the new 2D catalyst. Specifically, they found out how many neighboring atoms each co-catalyst has, how far away these neighbors are, and how they are connected to each other. The next stop in the investigation was NSLS-II’s Tender Energy X-ray Absorption Spectroscopy (TES) beamline.
Based on their experimental findings, the team used density functional theory to understand the structures and the mechanisms that control the efficiency of the reaction. They were able to validate their findings and the reaction mechanism. Only by combining their expertise in synthesis, analytical experimentation, and theoretical simulation could the team create their new 2D catalyst and demonstrate its efficiency.
Download the research summary slide
Related Links
Feature Story: “Two is Better Than One”
Contact
Jae-Hong Kim
Yale University
Jaehong.kim@yale.edu
Publication
C. Chu, Q. Zhu, Z. Pan, S. Gupta, D. Huang, Y. Du, S. Weon, Y. Wu, C. Muhich, E. Stavitski, K. Domen, J. Kim. Spatially separating redox centers on 2D carbon nitride with cobalt single atom for photocatalytic H2O2 production. Proc Nat Acad Sci 117 (12) 6376-6382 (2020). DOI: 10.1073/pnas.1913403117
Funding
This work was partially supported by National Science Foundation (NSF) Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment (Grant EEC-1449500). C.C. was financially supported by an Early Postdoctoral Mobility Fellowship, Swiss National Science Foundation (Award P2EZP2_168796) and D.H. was supported by the China Scholarship Council. This research used beamlines 8-BM (TES) and 8-ID (ISS) of the NSLS-II, US Department of Energy (DOE) Office of Science User Facilities operated for the DOE Office of Science by BNL under Contract DE-SC0012704. Computational work used the Extreme Science and Engineering Discovery Environment, supported by NSF (Grant ACI-1548562), through the Bridges high-performance computer at the Pittsburgh Supercomputing Center (Allocation ECD190001).
2020-18949 | INT/EXT | Newsroom









