Mesoporous Co/CeO2 Catalysts for Hydrogen Production
Scientists open a pathway for developing new and inexpensive catalysts
May 31, 2018
 enlarge
enlarge
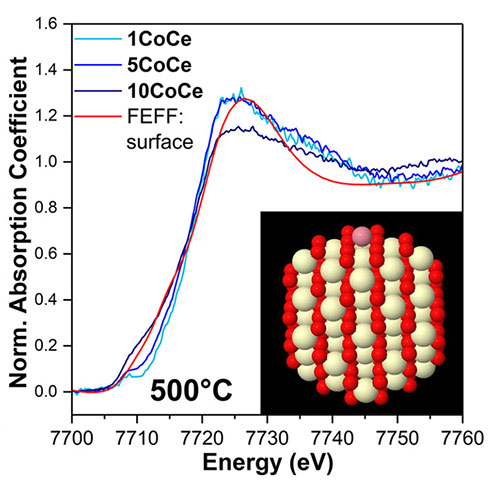
The image shows the x-ray absorption measurement of cobalt (Co) and a model of a Co atom (pink) on top of a ceria (CeO2) particle (yellow/red). Image credit: J. Phys. Chem. C ;122, 8998−9008 (2018)
The Science
Scientists characterized mesoporous cobalt/ceria (Co/CeO2) catalysts and found the catalysts to be active for water-gas shift reactions at 300 – 500 °C.
The Impact
The strong interactions seen between mesoporous ceria (CeO2) and cobalt (Co) may aid in the development of new and inexpensive catalysts through multimodal materials design.
Summary
Production of pure hydrogen gas for renewable fuel and chemical synthesis applications remains an area of significant research interest. Currently, over 95% of the world’s hydrogen supply is produced through the reforming of fossil fuels. This reforming process generates a product stream with a significant concentration of carbon monoxide (CO). The CO content is highly detrimental to the application of hydrogen toward synthesis processes and hydrogen fuel cells, since CO acts as a catalyst poisoning agent. For this reason, the removal of CO from reformate gas feeds is a necessary part of the hydrogen generation process.
The water-gas shift reaction is widely applied to industrial hydrogen generation processes to remove CO from reformate gas and generate additional hydrogen product. This reaction is typically carried out with the help of heterogeneous catalyst materials in two temperature stages to take advantage of both the kinetic and thermodynamic properties of the mildly exothermic reaction.
In this study, scientists examined mesoporous Co/CeO2 materials as a potential catalyst to foster the water-gas shift reaction. The researchers demonstrated that their catalysts showed significant catalytic activity for the high temperature region of the water-gas shift reaction. Their catalysts are small clusters, or atoms of cobalt, which are embedded into the mesoporous ceria preventing the typical chemistry seen for bulk Co.
The scientists used multiple characterization techniques, including x-ray imaging, scattering, and spectroscopy at various synchrotron-based instruments, including the Inner-Shell Spectroscopy (ISS) beamline and the In Situ and Operando Soft X-ray Spectroscopy (IOS) beamline at the National Synchrotron Light Source II (NSLS-II). NSLS-II is a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory.
The scientists found that the strong interactions seen between mesoporous ceria and cobalt may aid in the development of new and inexpensive catalysts through evidence-based materials design and synthesis. The reaction environment characterization of these low cobalt loading catalysts is a part of an ongoing effort to foster an understanding of the structural, chemical, and mechanistic properties of catalytic materials for industrially important reactions like the water-gas shift.
Download the research summary slide
Contact
Steven Suib
University of Connecticut
Steven.suib@uconn.edu
Sanjaya D. Senanayke
Brookhaven National Laboratory
ssenanay@bnl.gov
Publication
D. Vovchok, C Guild, S. Dissanayake, J. Llorca, E. Stavitski, Z. Liu, R. Palomino, I. Waluyo, Y. Li, A. Frenkel, J. Rodriguez, S. Suib, S. Senanayake. In Situ Characterization of Mesoporous Co/CeO2 Catalysts for the High-Temperature Water-Gas Shift. J. Phys. Chem. C 122, 8998−9008 (2018). DOI:10.1021/acs.jpcc.8b01271
Funding
The research carried out at Brookhaven National Laboratory was supported by the U.S. Department of Energy, Office of Science and Office of Basic Energy Sciences under Contract No. DE-SC0012704. This research used resources of the 8-ID (ISS) and 23-ID-2 (IOS) beamlines of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. This work also used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. J.L. is a Serra Hunter Fellow and is grateful to the ICREA Academia Program and MINECO/FEDER Grant ENE2015-63969-R and GC 2017 SGR 128. S.L.S. acknowledges support from the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Chemical, Biological and Geological Sciences (Grant DE-FG02-86ER13622A00) as well as the assistance of the Bioscience Electron Microscopy Laboratory of the University of Connecticut and Grant # 1126100 for the purchase of the FEI NovaSEM. A.I.F. acknowledges support from the U.S. Department of Energy, Office of Basic Energy Sciences Division of Chemical, Biological and Geological Sciences (Grant DE-FG02-03ER15476).
2018-18957 | INT/EXT | Newsroom