Research Highlight: Tripod Nanocatalysts Support Faster Fuel Cell Reactions
innovation at CFN
August 28, 2018
What is the scientific achievement?
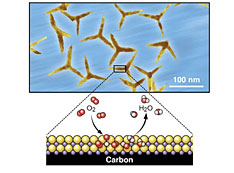
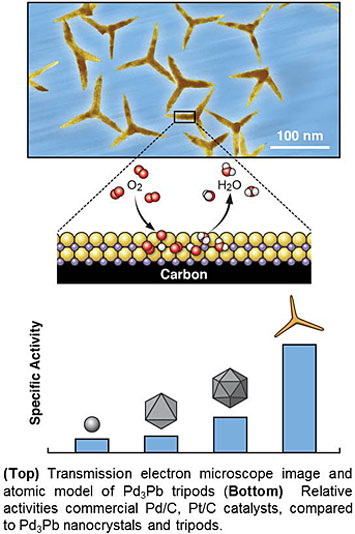
Highly efficient catalysts for the oxygen-reduction reaction (ORR) are important for fuel cell performance. Platinum (Pt)-based catalysts have shown the best performance, but it is desirable to replace them because of the high cost and relative scarcity of Pt. Scientists at the CFN and collaborators from Hong Kong Polytechnic University, Peking University, and Soochow University have designed and characterized palladium-lead (Pd3Pb) catalysts shaped like tripods. These catalysts show improved catalytic activity for the ORR as compared to commercial Pt/carbon (C) and Pd/C catalysts, and they maintain high performance over more than 20,000 cycles.
Why does this achievement matter?
This work demonstrates the promise of rationally designed high-performance catalysts through the appropriate control of nanomaterial facets and electronic structures.
What are the details?
CFN Capabilities: CFN Electron Microscopy facilities were used to characterize catalyst structure.
Highly efficient ORR catalysts are key to developing high-performance fuel cells and metal-air batteries. Though ORR catalysts based on Pd have the potential to replace those based on the more expensive and rare Pt, they typically have much lower ORR electrocatalytic activity compared to their Pt counterparts. In this work, the CFN—in collaboration with Hong Kong Polytechnic University, Peking University, and Soochow University—reported a class of structurally ordered Pd3Pb tripods with predominantly {110} exposed crystal facets. These tripods show extremely high activity and stability for the ORR in alkaline conditions. First-principles calculations suggest that a strong charge exchange between Pd-4d and Pb-(sp) orbitals in the {110} facets results in a Pd-Pb local bonding unit with an orbital configuration similar to that of Pt. As a consequence, Pd3Pb tripods exhibit much higher specific and mass activities for the ORR than either commercial Pt/C or Pd/C catalysts. The Pd3Pb tripods are durable for the ORR, sustaining more than 20,000 potential cycles under alkaline conditions, with negligible structural and compositional changes.
Publication Reference
“Coupled s-p-d Exchange in Facet-Controlled Pd3Pb Tripods Enhances Oxygen Reduction Catalysis”
L. Bu1, Q. Shao1, Y. Pi1, J. Yao1, M. Luo2,3, J. Lang1, S. Hwang4, H. Xin4, B. Huang5*, J. Guo6, D. Su4*, S. Guo2,3*, and Xiaoqing Huang1*
1 College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Jiangsu 215123 China
2 Department of Materials Science and Engineering, College of Engineering, Peking University, Beijing 100871, China
3 College of Engineering, Innovation Center for Engineering Science and Advanced Technology, Peking University, Beijing 100871, China
4 Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton, New York, USA
5 Department of Applied Biology and Chemical Technology, Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong Special Administrative Region, China
6 Testing & Analysis Center, Soochow University, Jiangsu 215123, China Chem 4 (2), 359–371 (2018)
Acknowledgement of Support
This work was financially supported by the Ministry of Science and Technology (2016YFA0204100, 2017YFA0208200), the National Natural Science Foundation of China (21571135 and 51671003), the National Key Research and Development Program of China (No. 2016YFB0100201), Young Thousand Talented Program, the start-up supports from Soochow University, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). Part of the electron microscopy work was performed at the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is supported by the U.S. Department of Energy (DOE), Office of Basic Energy Science, under contract DE-SC0012704.
2018-13089 | INT/EXT | Newsroom