- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events
Artificial Photosynthesis
We study light absorption and charge separation by band-gap narrowed semiconductors (BGNSCs) and transition metal complexes as chromophores replacing chlorophylls and the natural light-collecting and charge separation system in PS I and II. We investigate electron transfer from photosensitizers to catalysts for carrying out proton-coupled fuel generation reactions. We study how the holes produced by these charge separation events can oxidize water using molecular and heterogeneous catalysts to produce oxygen and protons. We also design and characterize systems for incorporating the electrons from the charge separation events into metal- and carbon-based hydrides analogous to NAPDH for carrying out reduction reactions, and design catalysts for carrying out fuel forming half-reactions analogous to the formation of carbohydrates in the Calvin cycle of PS I. The target reduction product might be either H2 or a reduced CO2 product. While reduced metal catalysts we are investigating can carry out two-electron reduction of CO2 to CO or formate, another promising route to a carbon-based fuel is to first produce H2 by a solar driven process involving a non-precious-metal-based hydrogen evolution catalyst (e.g., NiMoNx nanosheets on a carbon electrode support), and then using a catalyst such as [Cp*Ir(OH2)]2(THBPM) to reversibly convert the H2 and CO2 into an aqueous formate solution for use either in a formic acid fuel cell or as an efficient hydrogen storage and transport system.
We envision one possible integration of these components into an artificial photosynthetic process to be as shown in the diagram at right, where a light absorbing semiconductor material (a), in this arrangement, a photoanode) is shown in orange on the right-hard side of a photoelectrochemical cell. This photoanode has a water oxidation catalyst (b), represented by purple balls, attached to it. The so-called “oxidation half-reaction” (c) takes place at the catalyst on the photoanode. The other part of the cell contains a catalyst (d, pink balls) deposited on the cathode (e, shown in green) and caries out reduction half-reactions (f), such as reducing the protons produced in the water oxidation half-reaction at the photoanode or reducing dissolved CO2 in the solution.
Why do we need solar energy?
H2O and CO2 are stable end products (the end products of the combustion of fossil fuels) - renewable energy is needed to activate them.
Why do we use a photoanode?
Use photoinduced holes and electrons for water oxidation and proton (or CO2) reduction, respectively.
Why do we need catalysts?
Catalysts are necessary to promote multi-electron and multi-proton processes. They also allow achieving a lower overpotential and/or activation barrier for chemical transformations.
Why do we investigate water oxidation?
Fuel generation reactions have to be coupled with oxidative reaction to close catalytic cycles. Water is the only rational chemical to oxidize. The protons produced in the oxidation of water are used along with the electrons produced in the charge separation events in the reduction half-reactions. Water oxidation currently limits overall water splitting efficiency, and the O2 evolution mechanism still remains unclear .
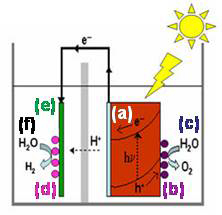
Above: an artificial photosynthetic process




