- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Catalysis: Reactivity and Structure
In situ studies of CeO2-supported Pt, Ru, and Pt-Ru alloy catalysts for the water-gas shift reaction: Active phases and reaction intermediates
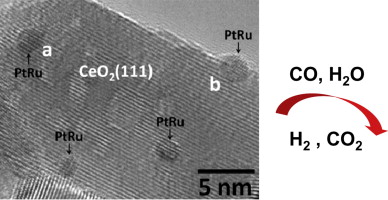
The activity and structure of three CeO2-based catalysts (Pt–CeO2, Ru–CeO2, and Pt–Ru alloy-CeO2) active for water–gas shift reaction (WGSR) are studied by in situ X-ray diffraction (XRD), operando X-ray absorption near edge spectroscopy (XANES), operando diffuse reflectance infrared Fourier-transform spectroscopy (DRIFTS), and high-resolution transmission electron microscopy (HRTEM) in order to understand and thus correlate both bulk and surface dynamics with performance. All systems investigated displayed a high WGS activity. Temperature-resolved XRD (under CO or H2 environment) indicated an additional expansion of the CeO2 crystal lattice at 100–250 °C for the catalysts compared to pure CeO2. This extra lattice expansion is due to additional oxygen removal from CeO2 promoted by the deposited Pt, Ru, or Pt–Ru alloy particles; CO showed an accelerated expansion when compared to that of H2. DRIFTS spectra revealed the formation of substantial amounts of formates (HCOO–) on Pt–CeO2 during WGSR, while formates on PtRu–CeO2 and Ru–CeO2 were at a much lower level. For all catalysts, formate species totally disappeared by 350 °C. The inhibition of formate formation on PtRu–CeO2 points to a modification of the chemical properties of Pt by alloying with Ru. The fact that the inhibition of Pt-bound formate species does not affect the catalytic activity implies that they are probably merely spectators on Pt, or at least not involved in the main reaction pathway. While the Pt–Ru alloy was not more active than Pt–CeO2, the alloyed catalyst did show a reduced generation of methane under WGSR conditions compared to Ru–CeO2. Pt XANES data confirmed the reduction of Pt in both Pt–CeO2 and PtRu–CeO2 with increasing temperature in the WGSR environment. HRTEM showed that the reduced PtRu–CeO2 catalyst was composed of a Pt–Ru alloy with a mean particle size of 2 nm well dispersed over the CeO2 support. Overall, the work indicates that a Pt–Ru alloy supported on CeO2 is an active and selective catalyst for WGSR.
Ref: Xu, W.Q., Si, R., Senanayake, S.D., Llorca, J., Idriss, H., Stacchiola, D., Hanson, J.C., and Rodriguez, J.A. Journal of Catalysis, 2012. 291: p. 117-126. DOI: 10.1016/j.jcat.2012.04.013




