- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Electrochemical Energy Storage
Using a novel characterization technique combining in situ X-ray diffraction and chemical sodiation to study the Li4Ti5O12 as new anode material for Sodium-ion batteries
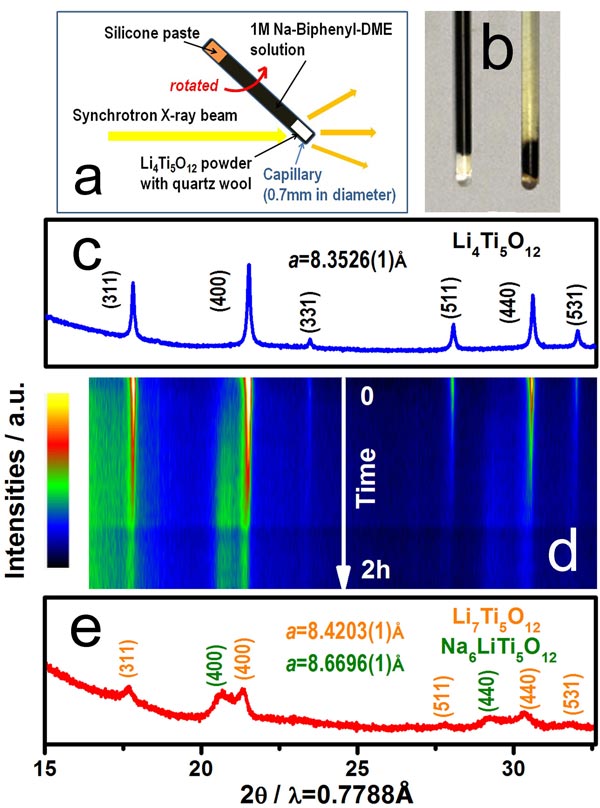
A novel characterization technique using the combination of chemical sodiation and synchrotron based in situ X-ray diffraction (XRD) has been developed. The power of this new technique was demonstrated in elucidating the structure evolution of Li4Ti5O12 upon sodium insertion. The sodium insertion behavior in Li4Ti5O12 is strongly size dependent. A solid solution reaction behavior in a wide range has been revealed during sodium insertion into the nano-sized Li4Ti5O12 (~44 nm), which is quite different from the well-known two-phase reaction of Li4Ti5O12/Li7Ti5O12 system during lithium insertion, and also has not been fully addressed in the literature so far. Based on this in situ experiment, the apparent Na+ ion diffusion coefficient (DNa+) of Li4Ti5O12 was estimated in the magnitude of 10-16 cm2 s-1, close to the values estimated by electrochemical method, but 5 order of magnitudes smaller than the Li+ ion diffusion coefficient (DLi+~10-11 cm2 s-1), indicating a sluggish Na+ ion diffusion kinetic in Li4Ti5O12 comparing with that of Li+ ion. Therefore, preparing the Li4Ti5O12 cathode with nano-sized particles is critical to make it a suitable anode material for sodium-ion batteries. The application of this new in situ chemical sodiation method developed by our group provides a facile way and a new opportunity for in situ structure investigations of various sodium-ion battery materials and other systems.
“A size-dependent sodium storage mechanism in Li4Ti5O12 investigated by a novel characterization technique combining in situ X-ray diffraction and chemical sodiation,” X. Yu, H.L. Pan, W. Wan, C. Ma, J. Bai, Q.P. Meng, S.N. Ehrlich, Y.S. Hu, X.-Q. Yang, Nano Lett. (2013), 13, 4721.




