- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Electrochemical Energy Storage
Determining the Key factor and element responsible to the slow kinetics in multi-element, high energy density cathode material for Li-ion batteries
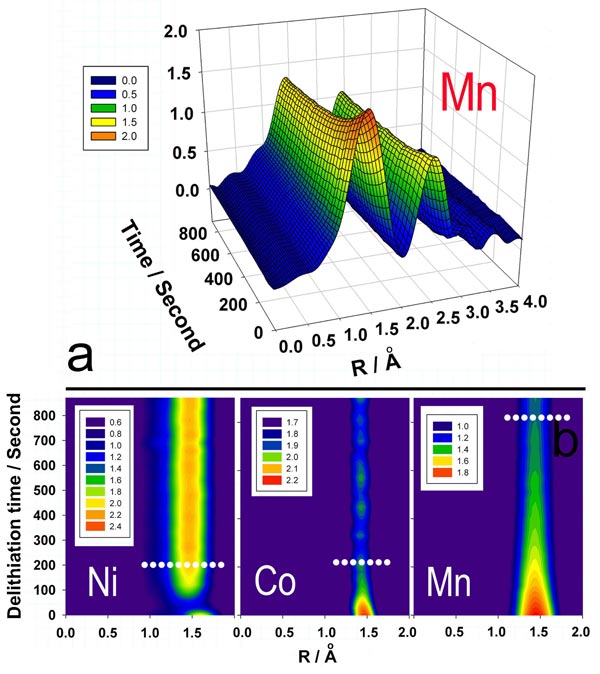
A new synchrotron based time resolved X-ray absorption spectroscopy (XAS) has been developed and applied to Li-ion cathode studies. Fourier transformed Extended x-ray absorption fine structure EXAFS spectra of Li1.2Ni0.15Co0.1Mn0.55O2 were measured during constant voltage charging at 5V. Ni, Co, and Mn were found to react simultaneously. A projection view of corresponding Ni-O, Co-O, Mn-O peak magnitudes of the Fourier transformed K-edge spectra as functions of charging time are plotted above. The reactions at Ni sites were completed in the first 180 seconds; in contrast, the reactions at Mn site were still going on even after 800 seconds, showing much slower delithiation kinetics around Mn sites. These results show that Mn is the limiting element for poor reaction kinetics.
“Understanding the rate capability of high-energy-density Li-rich layered Li1.2Ni0.15Co0.1Mn0.55O2 cathode materials,” X. Yu, Y. Lyu, L. Gu, H.M. Wu, S. Bak, Y. Zhou, K. Amine, S.N. Ehrlich, H. Li, K.W. Nam, X.-Q. Yang, Adv. Energy Mater. (2014), 4, 1300950.




