- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Surface Electrochemistry and Electrocatalysis
Electrochemical deposition of NPs, nanowires and refractory metal alloy cores; 100% utilization of Pt
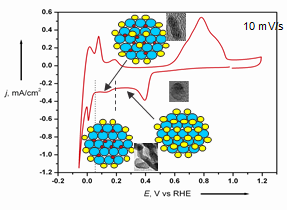
Role of Hads and Cl-ads in Pd nanowires, nanorods and nanoparticles growth on C.
It is generally accepted that the shape and composition of nanoparticles play a crucial role in determining their catalytic activity. Consequently, the shape and composition of cores will affect the core-shell interaction, i.e. the catalytic properties of a PtML shell. We recently demonstrated the possibility of obtaining by electrochemical deposition on carbon fibers and functionalized carbon nanoparticles two interesting types of cores: i) Pd NWs, and nanorods, ii) nanoparticles of refractory metal alloy (W + Ni) from aqueous solutions. This is a simpler procedure than various chemical routes. A co-deposition of W and Ni can take place in aqueous solutions. These particles, as cores, can provide a stable support (W passivates at high potentials and preclude dissolution of Ni from inner core). In addition, these are inexpensive cores. This is the first report of unidirectional metal growth on carbon nanoparticles.
The mechanism of this unexpected growth involves enhanced growth at nanoplanes induced by Hads, and suppressed growth at low-oordinated sites blocked by adsorbed chlorides. Each of these types of cores can subsequently be covered by a controlled PtML through a following electrodeposition. The unique feature of the electrodeposited PtML catalysts on electrodeposited cores on a gas diffusion layer (GDL) is that all Pt atoms are accessible to both electrons and protons and thus, they have a 100% utilization.
J. Electrochem. Soc.,




