- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Surface Electrochemistry and Electrocatalysis
Hollow core supported Pt monolayer catalysts
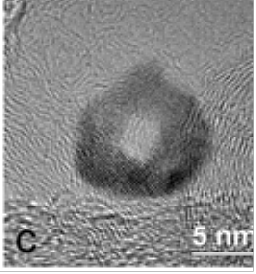
Hollow Pd/C nanoparticle
Another possibility to reduce O-BE involves the use of hollow cores. Recently we demonstrated an enhanced ORR activity of Pt hollow nanoparticle catalysts compared to solid ones. In order to further reduce the Pt content we developed PtML on hollow Pd or Pd20Au nanoparticle electrocatalysts. A hollow architecture of the Pd20Au particles was achieved by the delicate balance between galvanic displacement of Ni nanoparticles and the Kirkendall effect in controlling reaction kinetics. We ascribe the enhanced Pt mass and total-metal mass activities of PtML catalysts supported on hollow cores to the smooth surface morphology of the PtML catalyst supported on hollow Pd-based cores is evident in the TEM images.
In addition, lattice contraction was found for PtML on hollow cores by X-ray powder diffraction measurements, consistent with previous results with hollow Pt particles. Diffraction peaks shifts indicate a smaller lattice spacing of Pt was induced by hollow cores. The compressive strain was known to cause a down-shift of the d-band center of the PtML that result in a weaker Pt-OH interaction adds to higher ORR activity. Pt monolayer catalysts supported on such hollow cores exhibited total-metal mass activities ranging from 0.41 to 0.57 A mg−1, doubling that of 0.25 A mg−1 for Pt monolayer catalysts on solid Pd cores. We attribute this enhanced activity to the smooth surface morphology and hollow-induced lattice contraction, in addition to the mass-saving geometry of hollow particles.
Catalysis Today, 202 (2013) 50-54.




