Understanding Critical Failure in Li-S Pouch Cells
Scientists scale promising lithium-sulfur (Li-S) batteries to understand their current limitations
January 31, 2021
 enlarge
enlarge
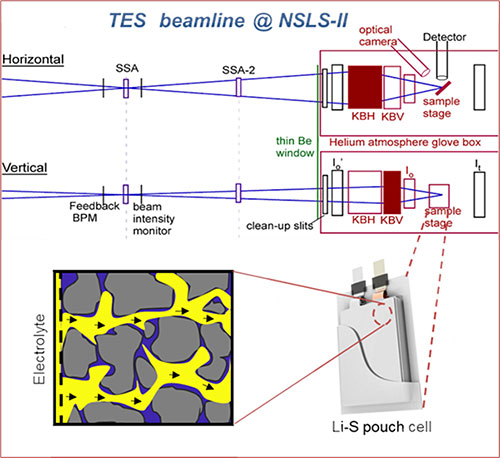
The schematics depict the reaction heterogeneity of high-mass-loading sulfur electrodes in practical Li-S pouch cells. Image credit: Xiao-Qing Yang and Jun Liu.
The Science
Scientists used state-of-the-art characterization tools to discover the reason for the catastrophic failure of a high-energy lithium-sulfur (Li-S) pouch cell and proposed stabilization strategies.
The Impact
Li-S batteries are a promising, sustainable energy storage technology. However, their current limitations are not well understood. This work offers new insights into Li-S battery limitations for scalable applications.
Summary
As global energy consumption rises, lithium–sulfur (Li–S) batteries pose a promising, sustainable energy storage technology due to their high theoretical energy and their use of low-cost materials. However, a realistic pouch cell demonstration is missing for commercialization of this technology.
In this study, researchers revealed new information about the cell failure mechanisms in pouch cells that can then assist in the strategic design of cell components and structure, as well as improve cell energy and extend cycle life. The researchers analyzed important parameters commonly used in coin cells and compared them with those needed for high-energy pouch cells. Based on the results of their analysis, they designed and fabricated pouch cells. The cells were analyzed using multiple characterization techniques and theoretical simulations of fluid flow. Among other methods, the team performed x-ray fluorescence microscopy (XRF) and x-ray absorption spectroscopy (XAS) experiments at the Tender Energy X-ray Absorption Spectroscopy (TES) beamline at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory.
The scientists found that limited diffusion causes uneven sulfur/polysulfide reactions and electrolyte depletions across the electrode leading to cell failure. They captured uneven reactions in multiple places within the pouch cell and their fluid flow simulations indicated that the electrolyte deficiency originates from the center of the cathode and then spreads to the edges.
The team suggested that new materials and strategies are required to produce Li–S cells that have both high specific energies and long cycle lives. In their proposed strategies, the scientists focus on increasing the supply of electrolytes and the rate of the electrolyte diffusion.
The researchers concluded that a full understanding of the interactions of cell components will be critical to unlock the full potential of Li–S battery technologies for commercialization.
Download the research summary slide
Contact
Dongping Lu
dongping.lu@pnnl.gov
Pacific Northwest National Laboratory
Jie Xiao
jie.xiao@pnnl.gov
Pacific Northwest National Laboratory
Jun Liu
jun.liu@pnnl.gov
Pacific Northwest National Laboratory
Publication
L. Shi, S.-M. Bak, Z. Shadike, C. Wang, C. Niu, P. Northrup, H. Lee, A. Y. Baranovskiy, C. S. Anderson, J. Qin, S. Feng, X. Ren, D. Liu, X.-Q. Yang, F. Gao, D. Lu, J. Xiao, J. Liu Reaction heterogeneity in practical high-energy lithium–sulfur pouch cells. Energy & Environmental Science 13 (10), 3620-3632 (2020). DOI: 10.1039/d0ee02088e
Funding
This research was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the U.S. Department of Energy through the Advanced Battery Materials Research (BMR) Program (Battery500 Consortium and BMR under Contract No. DEAC02-05CH11231 for PNNL and DE-SC0012704 for BNL). PNNL is operated by Battelle under Contract No. DE-AC05-76RL01830 for the U.S. Department of Energy. This research used beamline 8-BM (TES) of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704.
2021-18842 | INT/EXT | Newsroom









