Scientists Resolve Origin of Perovskite Instability
Inorganic perovskites have the potential for creating highly efficient solar cells
September 30, 2020
 enlarge
enlarge
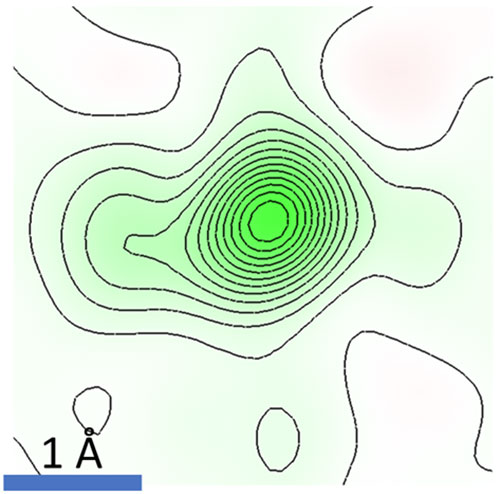
The room temperature cesium (Cs) electron density shows strong elongation – a signature of rattling. Image credit: D. B. Straus et. al. Adv. Mater. 32, 2001069 (2020).
The Science
Scientists have demystified the reason for the instability of halide perovskite CsPbI3.
The Impact
Halide perovskites, such as CsPbI3, have the potential for creating highly efficient solar cells. This work shows that the source of their thermodynamic instability is the cesium atom, suggesting new methods for engineering stable CsPbI3-based solar cells.
Summary
In recent years, researchers have worked to improve the efficiency of perovskite solar cells; however, some challenges remain before perovskite solar cells can become a competitive commercial product. One of these challenges is the instability of organic-inorganic hybrid perovskites, such as methylammonium lead iodide, thought to be related to the volatile nature of the organic cation. The all-inorganic perovskite cesium lead iodide (CsPbI3) does not have a volatile cation but is thermodynamically unstable, and the origin of the instability was not known to scientists until now.
In this work, researchers have demystified the reasons for instability in the inorganic perovskite cesium lead iodide (CsPbI3). To find the source of the instability, they investigated a sample over a wide range of temperatures from 100 to 295 K using single crystal x-ray diffraction at Princeton University and x-ray pair distribution function measurements performed at the Pair Distribution Function (PDF) beamline at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory.
Through their studies, the scientists discovered that the source of thermodynamic instability in the materials is the inorganic cesium atom and its “rattling” behavior within the crystal structure. The researchers concluded that while cesium remains on a single site within the structure at temperatures below 150 K, it inhabits or “splits between” two sites above 175 K.
These results reveal the limitations of tolerance factors in predicting perovskite stability and provide detailed structural information that suggests methods to engineer stable CsPbI3-based solar cells.
Download the research summary slide
Related Links
Feature Story: Lab Resolves Origin of Perovskite Instability
Contact
Daniel B. Straus
Princeton University
dstraus@princeton.edu
Robert J. Cava
Princeton University
rcava@princeton.edu
Publication
D. B. Straus, S. Guo, A. M. M. Abeykoon, R. J. Cava. Understanding the Instability of the Halide Perovskite CsPbI3 through Temperature-Dependent Structural Analysis. Advanced Materials 32, 2001069 (2020). DOI: 10.1002/adma.202001069
Funding
The synthesis of the compound and analysis of the diffraction data was supported by the Gordon and Betty Moore Foundation as part of the EPiQS initiative under Award No. GBMF-4412. The single crystal X-ray diffraction data collection was supported as part of the Institute for Quantum Matter, an Energy Frontier Research Center funded by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences under Award No. DE-SC0019331. This research used resources of the National Synchrotron Light Source II, a DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704.
2020-18943 | INT/EXT | Newsroom









