Single Atoms Break Carbon's Strongest Bond
Scientists demonstrate a new form of single-atom catalyst capable of breaking carbon-fluorine bonds, one of the strongest known chemical bonds
November 30, 2018
 enlarge
enlarge
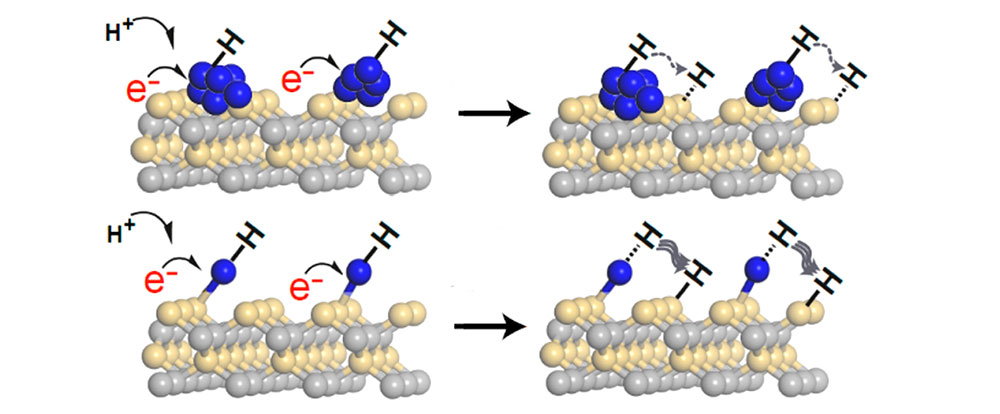
The image shows a direct comparison of the mechanisms of a platinum nanoparticle catalyst (upper row) and a single-atom platinum catalyst (lower row). The single-atom catalyst is more active because the hydrogen is only weakly bound and, therefore, can easily 'spill over' onto the silicon carbide surface. Image courtesy of D. Huang et. al., ACS Catalysis 8 (10), 9353-9358 (2018)
The Science
Scientists developed a new catalyst using single atoms of platinum for breaking carbon-fluorine bonds, one of the strongest known chemical bonds.
The Impact
Breaking carbon-fluorine (C-F) bonds is one of the most challenging reactions, and among the most important, in both chemical synthesis and environmental remediation of recalcitrant fluorinated hydrocarbons. A catalyst capable of breaking this bond could also be used to degrade polyfluoroalkyl substances (PFAS), one of the most challenging pollutant remediation problems of the present day.
Summary
Polyfluoroalkyl substances (PFAS) are one of the most challenging pollutants of the present day. They are widely detected all over the world, from Arctic biota to the human body, and their concentrations in contaminated groundwater significantly exceed the regulatory limit in many areas. Currently, there are no energy-efficient methods to destroy these contaminants.
In this work, scientists developed a new catalyst using single atoms of platinum (Pt) for breaking carbon-fluorine (C-F) bonds. Breaking the C-F bond offers a pathway to design a catalyst that is also capable of degrading PFAS.
The scientists used a facile, scalable, wet-chemical method to load single Pt atoms on a silicon carbide (SiC) surface. After loading the Pt atoms on the surface, they used two advanced x-ray characterization techniques at the Inner-Shell Spectroscopy (ISS) beamline to confirm the successful synthesis of Pt single atoms on the substrate. The ISS beamline is part of the advanced materials characterization capabilities at the National Synchrotron Light Source II’s (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory.
The team also compared Pt single atoms to Pt nanoparticle catalysts and found that the Pt single atoms are more reactive and selective than the Pt nanoparticles when catalyzing the chemical reaction for breaking C-F bonds, especially in H+ reduction step of the reaction. The scientists attributed this to the lower dissociation barrier of single-atom Pt, which only binds the hydrogen weakly and offers the possibility for an easy ‘spillover’ of the hydrogen onto the SiC surface. This spillover then promotes the breaking of C-F bonds.
Download the research summary slide
Related Links
Feature Story: "Single Atoms Break Carbon's Strongest Bond"
Contact
Jae-Hong Kim
Department of Chemical and Environmental Engineering, Yale University
jaehong.kim@yale.edu
Eli Stavitski
NSLS-II, Brookhaven National Laboratory
istavitski@bnl.gov
Publications
D. Huang, G. A. de Vera, C. Chu, Q. Zhu, E. Stavitski, J. Mao, H. Xin, J. A. Spies, C. A. Schmuttenmaer, J. Niu, G. L. Haller, J.-H. Kim,”Single-Atom Pt Catalyst for Effective C−F Bond Activation via Hydrodefluorination“, ACS Catalysis 8 (10), 9353-9358 (2018). DOI: 10.1021/acscatal.8b02660
Funding
This work was supported by the U.S. National Science Foundation (NSF) through the Nanosystems Engineering Research Center for Nanotechnology Enabled Water Treatment (Grant No. EEC-1449500), the China Scholarship Council (CSC), and an Early Postdoc Mobility Fellowship from the Swiss National Science Foundation (No. P2EZP2_168796). This research used beamline 8-ID (ISS) of the National Synchrotron Light Source II, and Center for Functional Nanomaterials U.S. Department of Energy (DOE) Office of Science User Facilities operated for the DOE Office of Science by Brookhaven National Laboratory (under Contract No. DESC0012704).
2018-18954 | INT/EXT | Newsroom