Revealing a Reaction Mechanism in an Electrode Material
Scientists studied how titanium disulfide in sodium (Na)-ion batteries could improve their performance for applications in electric vehicles
July 31, 2020
 enlarge
enlarge
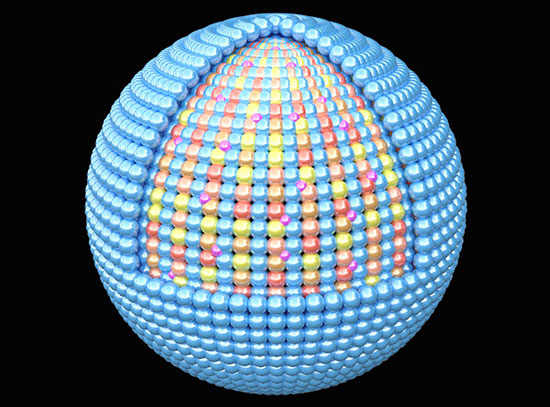
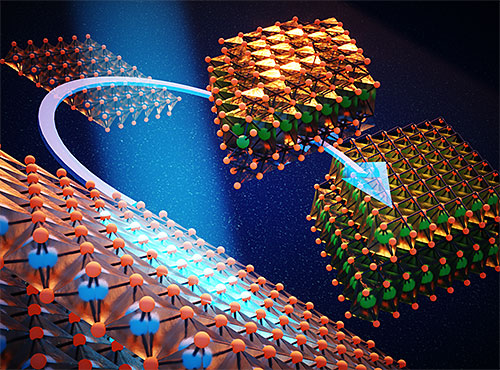
This work was selected to be highlighted on the front cover of the Journal of Materials Chemistry A. The cover image illustrates an artistic interpretation of the transitions between three phases during battery cycling. Image credit: C.-H. Lin, et. al. J. Mater. Chem A, 8, 12339-12350 (2020).
The Science
Scientists revealed the reaction mechanism occurring in Na–TiS2 batteries with a deeper understanding of the structural evolution.
The Impact
This mechanism may explain why Na-TiS2 has a better restoration of active materials, i.e. by restructuring after every cycle and potentially having better lifetime efficiency than lithium.
Summary
In recent years, the research interest and funding for large-scale energy storage systems for electric vehicles and intermittent sustainable energy for the electrical grid have increased, and the scientific community has explored a wide range of possible materials and solutions. However, a large research gap remains for current lithium-ion batteries to satisfy the demands of large-scale storage. Therefore, researchers are studying other options such as sodium (Na)-ion batteries.
Titanium disulfide (TiS2) is a promising candidate for electrode materials in Na-ion batteries due to its high electric conductivity, fast rate capability, and good cycling performance. Despite the well-studied electrochemical behaviors of TiS2 in Li-ion batteries, the detailed reaction mechanism of TiS2 in Na-ion batteries is not yet fully understood due to a more complex multiphase conversion process.
In this work, researchers investigated the reactions of TiS2 in Na-ion batteries via a multimodal approach using various x-rays techniques at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory. The team used the Tender Energy X-ray Absorption Spectroscopy (TES) beamline and the Inner-Shell Spectroscopy (ISS) beamline for operando x-ray absorption spectroscopy measurements and the X-ray Powder Diffraction (XPD) beamline for x-ray diffraction measurements.
By combining these techniques with computational modeling conducted at the Center for Functional Nanomaterials (CFN) —another DOE Office of Science User Facility at Brookhaven Lab—and computational resources from Brookhaven Lab’s’ Computational Science Initiative (CSI), the researchers discovered that the redox reaction in both titanium (Ti) and sulfur (S) during cycling has three structurally distinct phases. They further revealed that, due to changes in the titanium to titanium interatomic distances and behaviors, the battery does not fully recover after cycling.
Overall, the team presented a comprehensive analysis of the reaction mechanism occurring in Na–TiS2 batteries and provided a framework for studying a broader range of similar systems towards advanced energy storage and conversion applications.
Download the research summary slide
Contact
Karen Chen-Wiegart
Brookhaven National Laboratory/ Stony Brook University
Karen.Chen-Wiegart@stonybrook.edu
Publication
C.-H. Lin, M. Topsakal, K. Sun, J. Bai, C. Zhao, E. Dooryhee, P. Northrup, H. Gan, D. Lu, E. Stavitski, Y. K. Chen-Wiegart. Operando structural and chemical evolutions of TiS2 in Na-ion batteries. J. Mater. Chem. A, 8, 12339-12350 (2020). DOI: 10.1039/D0TA00226G
Funding
This work is supported by the U.S. Department of Energy (DOE) Office of Energy Efficiency and Renewable Energy under the Advanced Battery Materials Research (BMR) program, Contract No. DE-SC0012704. This research used resources 8-ID (ISS), 8- BM (TES) and 28-ID-2 (XPD) beamlines of the National Synchrotron Light Source II, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. This research used resources of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, and the Scientific Data and Computing Center, a component of the Computational Science Initiative, at Brookhaven National Laboratory under Contract No. DE-SC0012704.
2020-18800 | INT/EXT | Newsroom