- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Surface Electrochemistry and Electrocatalysis
We explore basic problems of electrocatalysis of fuel cell reactions, while focusing on platinum monolayer (PtML) electrocatalysts for the O2 reduction reaction (ORR), the electrocatalysts for ethanol and methanol oxidation to CO2, H2 evolution (HER) and H2oxidation (HOR) reactions. The goals include developing the fundamental understanding of these systems to allow syntheses of improved electrocatalysts with ultimately low Pt content. For the ORR that can be accomplished by fine-tuning of PtML shell properties in the interaction with cores of varying composition, shape and structure. We study hollow nanoparticle catalysts and cores, ultra-thin nanowire cores, and the stabilization of cores by alloying or by placing subsurface metal monolayers. The results will afford a viable approach for controlling chemical reactivity in the top atomic layer. Theoretical calculations are carried out for a deeper insight into the kinetics of these reactions, the role of proton transfer, and the bonding of O2, O, and OH. Studies using well-defined surfaces will provide understanding of the atomic-scale phenomena involved in core-shell interaction.
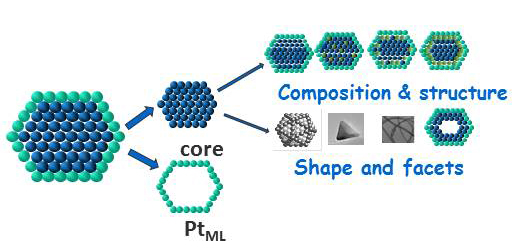
PtML electrocatalysts: Tuning properties by core-shell interaction.
For anodic fuel cell reactions our studies are focused on ethanol (ternary catalysts) and methanol (bifunctional catalysts) oxidation, and CO tolerant catalysts for H2 oxidation. We explore the catalysts capable of C – C bond splitting at low overpotentials and the catalysts for HER in water electrolysis. The success of our work will greatly contribute to resolving the major problems in existing fuel cell technology, and thereby opening the road to its successful commercialization.
The work of the EE group is funded by the U.S. Department of Energy Office of Science.
Group Leader
There are no people to show.