About the National Synchrotron Light Source II
From batteries and microelectronics to drug design and cultural heritage, scientists advance our understanding of many materials every day to solve the world’s greatest challenges. However, today's problems are so complex that they require scientists to study materials with highly specialized tools and expertise that are not readily available at most institutions.
That’s where the National Synchrotron Light Source II (NSLS-II) comes in. As one of the newest, most advanced synchrotron facilities in the world, NSLS-II enables its growing research community to study materials with nanoscale resolution and exquisite sensitivity by providing cutting-edge capabilities.
The facility’s state-of-the-art, medium-energy electron storage ring (3 billion electron-volts) generates ultrabright, highly stable beams of light, ranging from infrared to hard x-rays, to run 29 experimental stations, or beamlines. Each of our beamlines offers unique research tools to reveal the electronic, chemical, and atomic structure of materials.
Together with visiting researchers (users) and partners from all around the world, interdisciplinary teams at NSLS-II explore materials, focus on the most important challenges in science and technology. Learn more about this U.S. Department of Energy (DOE) Office of Science User Facility.

Have an idea for a new beamline? We would like to hear it. See this page for details.
NSLS-II News
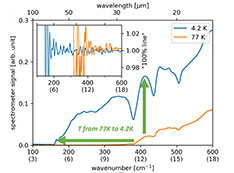
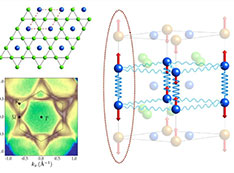
Science Highlights
Stay informed! Subscribe to our triannual newsletter, News @ NSLS-II.
Announcements
See all
Seminars / Events
- MAY1Wednesday
NSLS-II Public Tour
Public Tour of the National Synchrotron Light Source ll
Wednesday, May 1, 2024, 11 a.m., NSLS-ll Experimental Floor
- MAY22Wednesday
CBMS Lecture Series
Evaluation of Processing-Structure-Property Relationships of Lignocellulosic Biomass and Carbon-based Materials
Wednesday, May 22, 2024, 1:30 p.m., Videoconference / Virtual Event
Conferences / Workshops
Science Programs
Revealing the nano- to mesoscale structure and complex dynamics of heterogeneous systems under in situ conditions.
Characterizing and modeling the atomic structure and chemistry of complex materials during synthesis, processing, and energy conversion.
Imaging and quantifying the morphology, structure, chemistry, elemental variations, and strain distribution of materials.
Understanding and controlling the physics of emergent phenomena and catalysis at the nanoscale.
Partner Facilities

LBMS
Laboratory for BioMolecular Structure
Featuring state-of-the-art cryo-electron microscopes for life science imaging

CFN
Center for Functional Nanomaterials
A facility for exploring materials and processes at the nanoscale
User Services Office
Brookhaven National Laboratory
743 Brookhaven Avenue
Building 743
Upton, NY 11973-5000
(631) 344-8737 | nsls2user@bnl.gov | website
For Vendors & Contractors
If you are a contractor or vendor coming to NSLS-II for the day, please work with your host to gain access to the Lab site. If you will be on site for more than one day, please contact the Guest, User, and Visitor Center for access and training requirements. See maps and directions for getting here.