Efficient composite synthesis boosts battery energy delivery
October 23, 2017
What is the scientific achievement?
A team of users from the Stony Brook University m2M EFRC developed an efficient, ‘one-pot’ synthesis strategy for composite silver-iron battery materials, capable of delivering 2.4 times the energy density of the same composite formed by conventional mixing. Intimate contact between the two material components is the reason for the boost in energy density.
Why does this achievement matter?
New material solutions are required to meet the growing demand for energy storage. Composites can have desirable properties, but often lack efficient preparation methods. This scalable approach provides a potential solution using an environmentally abundant material (iron).
What are the details?
(Top) Silver-iron composites made by one-pot synthesis deliver 2.4 times the energy density of the same material made by conventional mixing. (Bottom Left) TEM image of the silver-iron composite battery material made by the new one-pot synthesis method. (Bottom Right) High resolution image showing intimate mixing of silver (red) and iron (green) in the composite. Each image represents an area of ~(200 nm)2.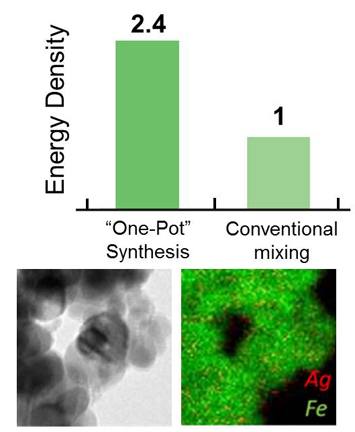 enlarge
enlarge
CFN Capabilities:
The CFN Electron Microscopy and Proximal Probes facilities were used to perform TEM and X-ray photoelectron spectroscopy measurements.
Publication Reference
J. L. Durham, C. J. Pelliccione, W. Zhang, A. S. Poyraz, Z. Lin, X. Tong, F. Wang, E. S. Takeuchi, A. C. Marschilok, K. J.Takeuchi, Silver ferrite/maghemite composites and mixtures: Impact of one-pot composite preparation on battery-relevant electrochemistry, Applied Materials Today, DOI:/10.1016/j.apmt.2016.12.003 (2017).
DOI: 10.1016/j.apmt.2016.12.003
Website:
dx.doi.org/10.1016/j.apmt.2016.12.003
Acknowledgement of Support
This work was supported as part of the Center for Mesoscale Transport Properties, an Energy Frontier Research Center supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, under award #DE-SC0012673. This research utilized XPS and TEM resources at the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. XAS data were collected at sector 12-BM at the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
2017-12586 | INT/EXT | Newsroom