Super-stable Platinum-alloy/Graphene-tube Catalysts
September 28, 2018
What is the scientific achievement?
A team of scientists from the CFN and the University of Buffalo developed and characterized a new Pt-alloy catalyst to promote the oxygen reduction reaction in fuel cells. This catalyst is formed by annealing Pt nanoparticles deposited onto graphene tubes co-doped with nitrogen, nickel, and cobalt. After annealing, the resulting Pt-alloy catalyst and N-doped graphene support enhanced catalytic activity and stability.
Why does this achievement matter?
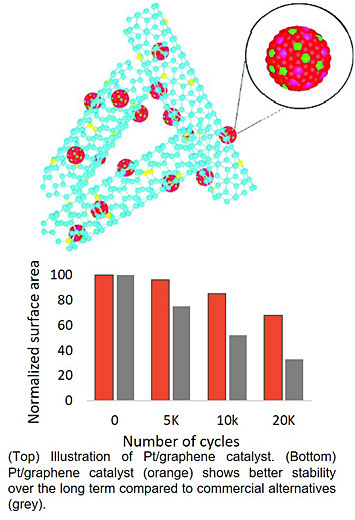
Pt catalysts on carbon supports are used to promote the oxygen reduction reaction in commercial fuel cells, but these catalysts suffer from poor long-term stability. The Pt-alloy catalysts synthesized in this work have excellent activity and improved stability, and represent a new design strategy exploiting a unique hybrid configuration.
What are the details?
In this work, a team of scientists from CFN, the University of Buffalo, and the University of South Carolina developed a highly active and stable Pt alloy catalyst supported by nitrogen/metal co-doped graphene tubes, targeting improvements in the oxygen reduction reaction for fuel cells. The Pt-M (Co, Ni) alloy is formed in-situ through diffusion of metals in the graphene tube during thermal annealing. The improved oxygen reduction reaction activity of these catalysts is attributed to formation of a Pt-M alloy phase, intrinsic active sites supplied by nitrogen/metal co-doped graphene tubes, synergistic effects between Pt and the nitrogen-doped carbon support, and enhanced mass transfer from appropriate mesopore/macropore distributions. The catalyst shows excellent stability at both low and high potential ranges. For example, the retained electrochemical surface area of the PtM/NGT catalyst is more than 2 times larger than that of the Pt/C catalyst after 20,000 potential cycles (0.6–1.0 V vs. RHE). Excellent long-term stability is ascribed to the highly graphitized carbon matrix, providing excellent support effect, mitigating the agglomeration of Pt nanoparticles, and preventing carbon corrosion.
CFN Capabilities:
The CFN Electron Microscopy Facility was used to characterize the catalyst structure.
Publication Reference
Mengjie Chen, Sooyeon Hwang, Jiazhan Li, Stavros Karakalos, Kate Chen, Yanghua He, Shreya Mukherjee, Dong Su, Gang Wu, Pt alloy nanoparticles decorated on large-size nitrogen-doped graphene tubes for highly stable oxygen-reduction catalysts, Nanoscale 10, 17318 (2018).
DOI:10.1039/C8NR05888A
http://pubs.rsc.org/en/Content/ArticleLanding/2018/NR/c8nr05888a#!divAbstract
Acknowledgement of Support
This work is financially supported by the start-up funding from the University at Buffalo, SUNY along with U.S. DOE-EERE Fuel Cell Technologies Office. Electron microscopy research was conducted at the Center for Functional Nanomaterials at Brookhaven National Laborat
2018-14306 | INT/EXT | Newsroom