Manganese Nanocatalysts Provide a Fuel Cell Boost
September 30, 2019
What is the scientific achievement?
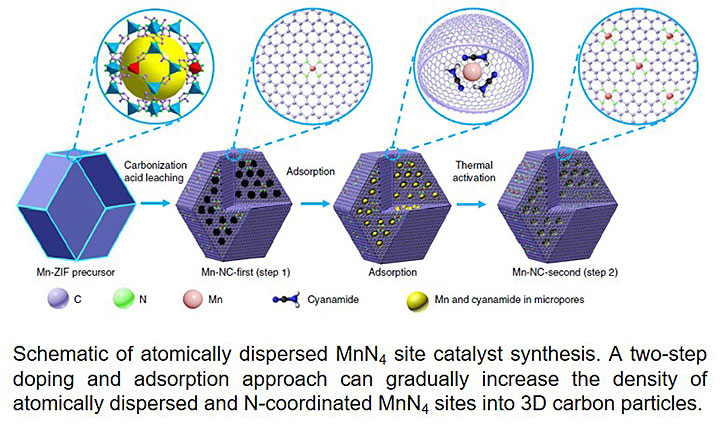
CFN and CNMS users from the University of Buffalo led a collaborative study of a new manganese catalyst that significantly enhances the important oxygen reduction reaction in fuel cells. The catalyst enables a large half-wave potential of 0.80 V and remains stable in acidic environments in which fuel cells operate. Mechanistic studies identify the 4-electron pathway responsible for the enhanced performance. The catalyst structure — atomically dispersed MnN4 embedded in graphitic carbon, was established by multimodal X-ray absorption spectroscopy and atomic resolution electron microscopy.
Why does this achievement matter?
Discovery of catalysts that are free from platinum group metals is necessary for wider adoption of fuel cell technologies. The catalyst structure and reaction mechanism identified here provide clues for progress on this important goal.
What are the details?
Platinum group metal (PGM)-free catalysts that are also iron free are highly desirable for the oxygen reduction reaction (ORR) in proton-exchange membrane fuel cells, as they avoid possible Fenton reactions. Here we report an efficient ORR catalyst that consists of atomically dispersed nitrogen-coordinated single Mn sites on partially graphitic carbon (Mn-N-C). Evidence for the embedding of the atomically dispersed MnN4 moieties within the carbon surface-exposed basal planes was established by X-ray absorption spectroscopy and their dispersion was confirmed by aberration-corrected electron microscopy with atomic resolution. The Mn-N-C catalyst exhibited a half-wave potential of 0.80 V versus the reversible hydrogen electrode, approaching that of Fe-N-C catalysts, along with significantly enhanced stability in acidic media. The encouraging performance of the Mn-N-C catalyst as a PGM-free cathode was demonstrated in fuel cell tests. First-principles calculations further support the MnN4 sites as the origin of the ORR activity via a 4e− pathway in acidic media.
CFN Capabilities
Electron Microscopy Facilities at the CFN and CNMS provided high-resolution transmission electron microscopy and scanning transmission electron microscopy.
Publication Reference
J. Li, M. Chen, D.A. Cullen, S. Hwang, M. Wang, B. Li, K. Liu, S. Karakalos, M. Lucero, H. Zhang, C. Lei, H. Xu, G.E. Sterbinsky, Z. Feng, D. Su, K.L. More, G. Wang, Z. Wang, G. Wu, Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nature Catalysts 1, 935 (2018).
https://www.nature.com/articles/s41929-018-0164-8
Nature News & Views Highlight:
FUEL CELLS, Atomic approaches towards stability Nature Catalysis 1, 900 (2018).
https://www.nature.com/articles/s41929-018-0205-3
Acknowledgement of Support
G. Wu thanks the Research and Education in eNergy, Environment and Water (RENEW) program at the University at Buffalo, SUNY and National Science Foundation (CBET-1604392, 1804326) for partial financial support. G. Wu, G. Wang and H.X. acknowledge support from the US Department of Energy (DOE), Energy Efficiency and Renewable Energy, Fuel Cell Technologies Office (DE-EE0008075). Electron microscopy research was conducted at Oak Ridge National Laboratory’s Center for Nanophase Materials Sciences of (D.A.C. and K.L.M) and the Center for Functional Nanomaterials at Brookhaven National Laboratory (S.H. and D.S., under contract No. DE-SC0012704), which both are US DOE Office of Science User Facilities. XAS measurements were performed at beamline 9-BM at the Advanced Photon Source, a User Facility operated for the US DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357 (Z.F. and G.E.S.). Z.W. and J.L. thank the National Natural Science Foundation of China (Grant No. 21273058 and 21673064) for support.
2019-16871 | INT/EXT | Newsroom