Uncovering Better Catalysts in Confined 2D Spaces
April 30, 2021
What is the scientific achievement?
CFN scientists demonstrated that nanoporous silica films less than one nm thick boost the activity of a palladium surface for carbon monoxide oxidation, a critical reaction in environmental chemistry. In situ IR & X-ray spectroscopies and mass spectrometry reveal that the film nanostructure disperses CO adsorption and restricts adsorption geometries, enhancing CO conversion and increasing CO2 production by 12 percent, compared to that on palladium alone.
Why does this achievement matter?
Carbon monoxide oxidation converts poisonous CO and O2 to CO2, often with the aid of catalytic palladium. The results reveal the impact of 2D silica films in enhancing metal-catalyzed reactions, which can be applied to other confined reactions and 2D materials.
What are the details?
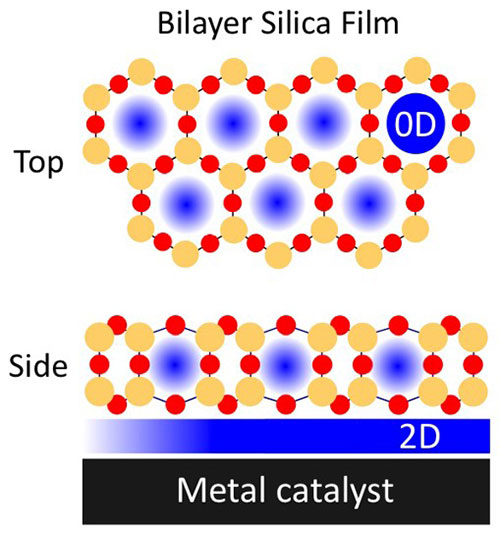
Interfacially confined microenvironments have recently gained attention in catalysis, as they can be used to modulate reaction chemistry. The emergence of a 2D nanospace at the interface between a 2D material and its support can promote varying kinetic and energetic schemes based on molecular level confinement effects imposed in this reduced volume. We report on the use of a 2D oxide cover, bilayer silica, on catalytically active Pd(111) undergoing the CO oxidation reaction. We “uncover” mechanistic insights about the structure-activity relationship with and without a 2D silica overlayer using in situ IR and X-ray spectroscopy and mass spectrometry methods. We find that the CO oxidation reaction on Pd(111) benefits from confinement effects imposed on surface adsorbates under 2D silica. This interaction results in a lower and more dispersed coverage of CO adsorbates with restricted CO adsorption geometries, which promote oxygen adsorption and lay the foundation for the formation of a reactive surface oxide that produces higher CO2 formation rates than Pd alone.
The results allowed the research team to elucidate the mechanistic parameters needed to improve CO2 efficiency. This effort was greatly enhanced by the use of the CFN Proximal Probes facility and the IOS beamline at NSLS-II. Working with CFN and NSLS-II scientists, the team measured the spectroscopy response of CO2 conversion at the functionalized and bare metal catalyst, which allowed for crucial understanding of the physics in the confined space.
CFN Capabilities
The CFN Proximal Probes Facility and IOS beamline at NSLS-II (a partnership between CFN and NSLS-II) were used to perform infrared reflection absorption spectroscopy, ambient-pressure X-ray photoelectron spectroscopy, and mass spectrometry.
Publication Reference
C.N. Eads,1 J.A. Boscoboinik,1 A.R. Head,1 A. Hunt,2 I. Waluyo,2 D.J. Stacchiola,1 S.A. Tenney,1 “Enhanced Catalysis under 2D Silica: A CO Oxidation Study”, Angew. Chem. Int. Ed. 60, 19 (2021).
DOI: https://doi.org/10.1002/anie.202013801
OSTI: https://www.osti.gov/biblio/1762146-enhanced-catalysis-under-silica-co-oxidation-study
CFN Newsroom: Chemistry Goes Under Cover
Acknowledgement of Support
This work used resources of the 23-ID-2 (IOS) beamline at the National Synchrotron Light Source II and the Center for Functional Nanomaterials, which are U.S. DOE Office of Science Facilities, at Brookhaven National Laboratory under Contract No. DE-SC0012704. J.A.B. and D.J.S. were supported by Integrated Mesoscale Architectures for Sustainable Catalysis (IMASC), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under award #DE-SC0012573.
2021-18980 | INT/EXT | Newsroom