Learning from Pileups in Li-Ion Batteries
May 28, 2021
 enlarge
enlarge
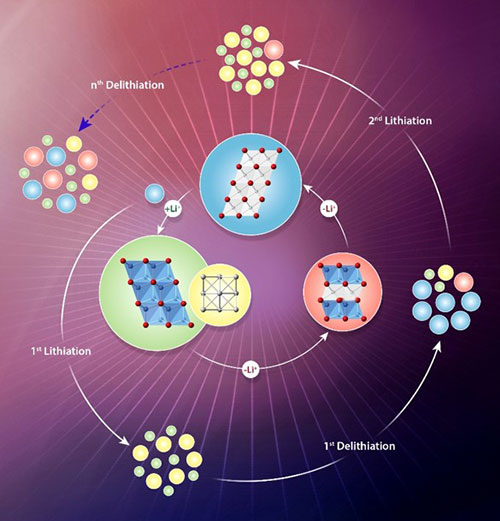
An illustration of metal-oxide electrode structural changes during battery cycling. The inner circle shows structural changes during lithium insertion/extraction. The outer circle shows how the material degrades by incomplete lithium removal reactions over several cycles.
What is the scientific achievement?
A collaborative team of scientists at Brookhaven Lab and Kyungpook National University used transmission electron microscopy and synchrotron x-ray techniques to understand the structural changes in metal-oxide battery electrodes during charging and discharging. They found asymmetric reaction pathways for lithium insertion (charging) and extraction (discharging), with slow lithium-ion diffusion and incomplete lithium extraction during discharge, accelerating the electrode degradation.
Why does this achievement matter?
A detailed understanding of how the structure of conversion-type metal oxide electrodes evolves during battery cycling can be applied to optimize the performance of other conversion-type materials like metal fluorides and sulfides.
What are the details?
Metal oxides have been actively explored as promising conversion-type electrode materials for lithium-ion batteries because they deliver high capacities. However, they suffer from poor cyclability, capacity fading, and voltage hysteresis. A fundamental understanding of these properties is lacking. In this work, multimodal analyses were performed to monitor the structural changes of metal oxides during lithium insertion and removal for several cycles. The analyses showed that phase transformation occurs in different ways—lithiation follows a kinetically driven route, while delithiation adopts a path close to the thermodynamic ground states. Asymmetric reaction routes are responsible for the voltage hysteresis. At the end of delithiation, an intermediate phase was found (Li-M-O), indicating lithium ions were not fully removed. Slow diffusion of metal ions and a high energy barrier for lithium extraction are mainly responsible for incomplete lithium extraction reaction. Imperfect lithium removal eventually results in the loss of active materials over the repeated cycling.
CFN Capabilities
The CFN Electron Microscopy Facility was used for characterization of the electrode materials during cycling, as were the PDF and XPD beamlines at NSLS-II.
Publication Reference
S. Li, Z. Shadike, G. Kwon, X.-Q. Yang, J. H. Lee, S. Hwang, Asymmetric Reaction Pathways of Conversion-Type Electrodes for Lithium-Ion Batteries, Chemistry of Materials, Accepted Publication (2021).
DOI: https://doi.org/10.1021/acs.chemmater.0c04466
Acknowledgement of Support
This work is supported by the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE- SC0012704. This research used the PDF and XPD beamlines (28-ID-1 and 28-ID-2) at the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. Z.S. and X.-Q.Y. at Brookhaven National Laboratory are supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Vehicle Technology Office of the U.S. Department of Energy through the Advanced Battery Materials Research (BMR) Program under contract DE-SC0012704.
2021-18982 | INT/EXT | Newsroom