Adding Up to Create Environmentally Friendly Anti-Corrosion Coatings
January 21, 2022
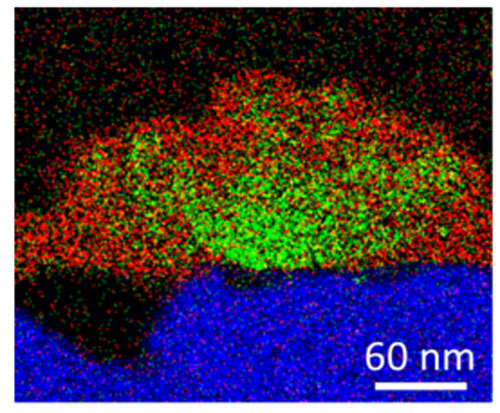 enlarge
enlarge
Cross-sectional energy-dispersive x-ray analysis map showing the elemental composition of an iron substrate (blue), and a corrosion coating containing copper (green) and zirconium (red).
What is the scientific achievement?
CFN users from Stony Brook University, Brookhaven Lab, and Henkel Corporation worked with CFN staff to understand the mechanistic benefits of organic (polyamidoamine) and inorganic (Cu) additives on environmentally friendly, zirconium-based (Zr-based) anti-corrosion coatings. Multimodal characterization by electron- and x-ray techniques characterized the structure and chemistry of the coatings, revealing that optimal amounts of each additive promotes improved morphology, uniformity, and substrate adhesion of the Zr-based coating.
Why does this achievement matter?
Corrosion is destructive to metals and alloys and causes substantial economic, health, and ecological loss. Conventional anti-corrosion coating materials are toxic and carcinogenic. This mechanistic understanding will inform development of environmentally friendly surface-treatment technologies optimized to inhibit corrosion in the automotive, aerospace, and energy storage industries.
What are the details?
Environmentally friendly, chromate-free zirconium (Zr)-based conversion coatings are a promising green technology for corrosion protection. Additives in the surface treatment provide critical functionalities and performance improvements; however, a mechanistic understanding of how the additives influence the coatings remains unclear.
In this study, the researchers present a hybrid Zr-based conversion coating that combines copper (Cu) compounds and polyamidoamine (PAMAM), taking advantage of the complementary nature of organic and inorganic additives. Using multimodal electron and x-ray characterization techniques—scanning electron microscopy (SEM), focused-ion beam/SEM, scanning transmission electron microscopy (STEM), and x-ray photoelectron spectroscopy (XPS) at the Center for Functional Nanomaterials (CFN) and x-ray absorption near-edge structure (XANES) spectroscopy at the NSLS-II Beamline for Materials Measurement (BMM)—they studied the interaction of Cu2+ and PAMAM and the resulting impacts on coating formation. Adding PAMAM changes the surface morphology, thickness, distribution of Cu in clusters, and void formation of the coatings. A high concentration of PAMAM (100–200 ppm) leads to little conversion coating formation, and a low concentration of PAMAM (0–25 ppm) shows voids forming under the coatings. Moreover, PAMAM incorporates in the coating in the form of a PAMAM-Cu complex with a higher concentration toward the surface, providing an organic layer at the coating surface. The contribution of PAMAM to the enhanced chemical stability of the coating in an alkaline environment is revealed by XANES spectroscopy. The team found an intermediate range of PAMAM additive (50 ppm) is optimal to avoid excessive void formation, promote some Cu cluster formation and thus enhance the Zr-based coating formation, and incorporate organic components into the coating to improve adhesion properties. This study furthers our knowledge of the formation mechanism of an effective and environmentally friendly hybrid conversion coating for corrosion inhibition, demonstrating a critical processing-structure-property relationship. The scientific insights provided here will inform the development of green surface-treatment technologies for the automotive, aerospace, and other industries.
CFN Capabilities
The CFN Nanofabrication, Electron Microscopy, and Proximal Probes Facilities were used to fabricate and characterize the anti-corrosion coatings.
Publication Reference
X. Liu, D. Vonk, K. Kisslinger, X. Tong, G. Halada, S. Petrash, K. Foster, Y. K. Chen-Wiegart. “Unraveling the Formation Mechanism of a Hybrid Zr-Based Chemical Conversion Coating with Organic and Copper Compounds for Corrosion Inhibition,” ACS Applied Materials & Interfaces 13, 4 (2021).
DOI: https://pubs.acs.org/doi/10.1021/acsami.0c19203
Acknowledgment of Support
This work was supported by Henkel Corp., Award 81113. This research used resources Beamline for Materials Measurement (BMM, 6-BM) of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) office of Science Users Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract DE-SC0012704. The authors are grateful to Dr. Bruce Ravel (National Institute of Standards and Technology), a scientist at BMM beamline, for his expertise and support on XANES characterization as well as his insights on data analysis and scientific interpretation. This research used resources of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract DE- SC0012704. X.L. and K.C.-W. acknowledge the support by the Department of Materials Science and Chemical Engineering, the College of Engineering and Applied Sciences, and Stony Brook University.
2022-19375 | INT/EXT | Newsroom









