Rapid Atomic Ordering for Catalytic Nanoparticles
January 21, 2022
What is the scientific achievement?
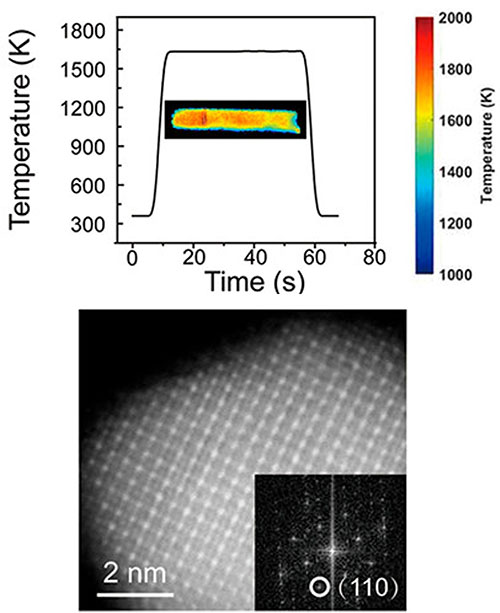 enlarge
enlarge
(Top) Temperature profile during Joule heating. (Bottom) Ordered Pd3Pb intermetallic nanoparticle
A collaborative team of scientists demonstrated a rapid Joule heating method to synthesize highly ordered, ultrasmall intermetallic nanoparticles. The short heat treatment (60 seconds) prevents particle aggregation and yielded nanoparticles (~6 nm) with a narrow size distribution, which showed excellent electrocatalytic activity and stability when catalyzing the oxygen reduction reaction.
Why does this achievement matter?
The work demonstrates a new synthesis route for small, highly ordered, well-dispersed intermetallic nanomaterials, and provides new avenues to catalyst discovery.
What are the details?
Intermetallic compounds, featuring well-defined stoichiometry and structural long-range atomic ordering, exhibit interesting properties compared to alloy compounds where atoms are located randomly. Catalysts with atomic ordering show promising catalytic behavior as well as stability. However, long heat treatments are usually used to achieve well-defined atomic ordering, which results in particle agglomeration and degradation of catalytic performance. This research demonstrates a Joule heating approach that can synthesize highly ordered intermetallic nanoparticles in a short period (~60 s). Rapid heating is effective to transform Pd and Pb metal precursors to Pd3Pb intermetallic nanoparticles with a L12 structure and a size of about 6 nm. Density functional theory calculations suggest that high concentration of vacancies generated at high temperature facilitate the atomic diffusion process in the crystalline state. Moreover, electrochemical tests validate excellent electrocatalytic activity and outstanding stability for oxygen reduction reaction (ORR) of ordered Pd3Pb particles.
CFN Capabilities
- Pd3Pb intermetallic nanoparticles were synthesized
- Joule heating to 1600 K with a ramp rate of 105 K s-1 was used for quick atomic ordering of the Pd3Pb
- The CFN Electron Microscopy Facility was used to characterize the ordered intermetallic nanoparticles
Publication Reference
M. Cui,# C. Yang,# S. Hwang,# B. Li, Q. Dong, M. Wu, H. Xie, X. Wang, G. Wang, L. Hu, “Rapid Atomic Ordering Transformation toward Intermetallic Nanoparticles” Nano Letters 22, 255 (2022).
#Contributed equally to the work
DOI: 10.1021/acs.nanolett.1c03714
OSTI: www.osti.gov/biblio/1838177-rapid-atomic-ordering-transformation-toward-intermetallic-nanoparticles
Acknowledgment of Support
We acknowledge the support of the Maryland Nanocenter, its Surface Analysis Center and AIMLab. We would like to thank Dylan Kline and Michael R. Zachariah at the University of Maryland, College Park for their assistance with the MATLAB coding for the high-temperature Joule heating measurement. The electron microscopy work used resources at the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. B.L. and G.W. gratefully acknowledge the financial support from the National Science Foundation (NSF) (DMR 1905572) and the computational resources provided by the University of Pittsburgh Center for Research Computing.
2022-19596 | INT/EXT | Newsroom









