More Hydrogen from "Hot" Water Splitting
July 29, 2022
 enlarge
enlarge
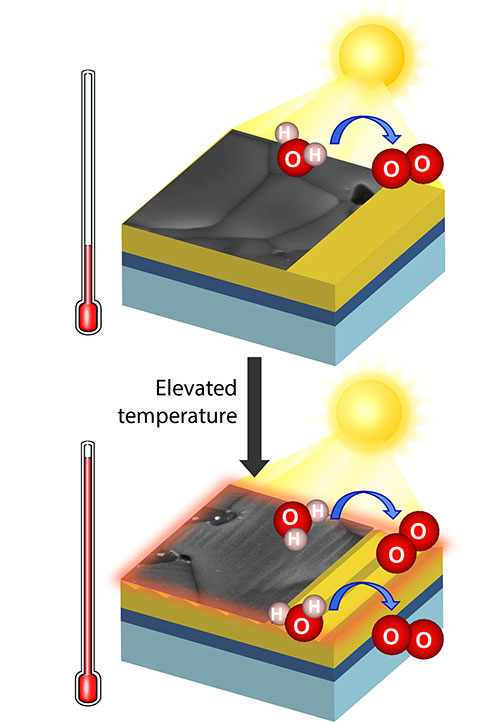
Photoelectrochemical reactions at elevated temperature generate favorable changes in the photoelectrode surface.
Scientific Achievement
CFN researchers demonstrated that elevated electrolyte temperatures increase the activity of a bismuth vanadate electrode for solar hydrogen generation by photoelectrochemical water splitting by 40 percent, due to more efficient charge carrier separation and a favorable semiconductor surface reconstruction.
Significance and Impact
This work provides insights on the effect of operating temperature on photoelectrode surface morphology and provides a new understanding of scavengers commonly used in photoelectrochemical studies.
Research Details
Photoelectrochemical (PEC) water splitting is typically studied at room temperature. In this work, the temperature effect on PEC water splitting is studied using a BiVO4 thin film photoanode as a model system. The systematic temperature-dependent electrochemical study demonstrates that the PEC activity is boosted at elevated electrolyte temperatures and indicates that thermal energy plays a main role in improving charge carrier transport in the bulk of BiVO4. Irreversible surface reconstruction is observed after PEC reactions at elevated temperature in the presence of hole scavengers, with regularly spaced stripes emerging on BiVO4 grains. The surface-reconstructed photoanode exhibits up to 40% improvement in photocurrent densities and ∼ 0.25 V shift of photocurrent onset to favorable direction. The detailed investigation shows the formation of an amorphous layer without stoichiometric change at the reconstructed surface. This work provides insights of the temperature effect on the photoelectrode in solar water splitting and reveals the effect of hole scavengers in photoelectrochemical measurement.
- The favorable, irreversible electrode surface reconstruction occurs via reactions with hole scavengers at elevated electrolyte temperatures.
- The CFN Proximal Probes Facility, Materials Synthesis & Characterization, and Electron Microscopy facilities were used for this study.
Publication Reference
C. Zhou, L. Zhang, X. Tong, M. Liu. “Temperature Effect on Photoelectrochemical Water Splitting: A Model Study Based on BiVO4 Photoanodes.” ACS Applied Materials & Interfaces 13, 61227 (2021).
DOI: https://doi.org/10.1021/acsami.1c19623
OSTI: https://www.osti.gov/biblio/1838175
Acknowledgment of Support
This research used resources of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science User Facility, at Brookhaven National Laboratory under contract no. DE-SC0012704.
2022-21053 | INT/EXT | Newsroom









