Detecting Pollutant Gases with Aromatic Sensors
January 31, 2023
 enlarge
enlarge
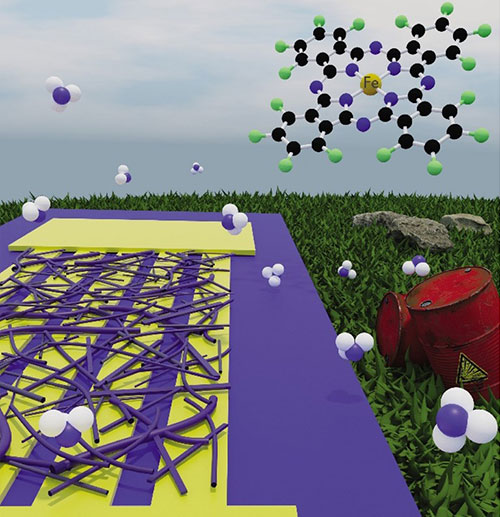
Illustration of a nanowire sensor monitoring ammonia levels in air. The top right corner shows the structure of a fluorinated metal phthalocyanine complex.
Scientific Achievement
Researchers from the University of Puerto Rico at Río Piedras have developed gas sensors based on metal phthalocyanine nanowires grown directly on electrical contacts. These sensors can detect ammonia in air at levels of 40 parts per billion (ppb) in measurement times of 2.5 hours, which is suitable for environmental monitoring.
Significance and Impact
These devices are well-suited for environmental sensing of toxic gases in recovery zones, due to their high sensitivity, reproducibility, recovery, stability, and very low power energy consumption (35 nW).
Research Details
The work describes the single-step production of gas sensing devices from unsubstituted and hexadecafluorinated metal phthalocyanines (MPc, M = Fe2+ and Co2+). The team prepared sensor devices by the direct growth of nanowires on interdigitated electrodes by the vapor transport of the synthesized MPc precursors. Results using as-prepared devices for the detection of NH3 and NO2 in the ppb range are part of the published work. In agreement with similar MPc sensing materials, response and recovery times were fitted using a double-exponential model that gave two rate constants: a short one, on the order of minutes for concentrations above 500 ppb, and a long one, on the order of hours. These rate constants are suitable for environmental monitoring of gases in recovery zones, where longer exposure times are critical in the sampling process. The fluorinated nanowire sensors showed a ∼10% normalized response toward NH3 at 40 ppb for a measuring time of ∼2.5 h at room temperature and measurable responses to concentrations as low as 5 ppb, rendering them applicable to environmental studies.
- Ammonia concentrations as low as 5 ppb were detected using longer exposures (~days).
- Detectors have no significant cross-sensitivity with other reducing gases tested.
- CFN Electron Microscopy (remote operation) and Nanofabrication Facility capabilities were used for this study.
CFN scientists characterized the nanowires using Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) with Energy Dispersive X-ray (EDX) Spectroscopy to obtain high-resolution images of the sensor nanowires for crystal phase identification and elemental mapping. Fernando Camino coordinated the collaboration between the Puerto Rico and CFN teams, performed SEM imaging (Helios G5 SEM/FIB) of the nanowires, and implemented the remote operation of the TEM (Talos F200X TEM). Kim Kisslinger obtained high-resolution TEM Images and realized crystal phase identification and elemental mapping of the nanowires.
Publication Reference
Flores S. Y., Gonzalez-Espiet J., J. Cintrón, N. De Jesus Villanueva, F. E. Camino, K. Kisslinger, D. M. Piñero Cruz, R. Díaz Rivera, L. F. Fonseca. “Fluorinated Iron and Cobalt Phthalocyanine Nanowire Chemiresistors for Environmental Gas Monitoring at Parts-perBillion Levels.” ACS Applied Nano Materials 5, 4688 (2022).
DOI: https://doi.org/10.1021/acsanm.1c04039
OSTI: https://www.osti.gov/biblio/1856772
Acknowledgment of Support
Funding for this research was provided by NSF-CREST CIRE2N (Grant Number HRD-1736093) and NSF (Grant Number CHE-1626103). R.D. acknowledges the ONR Summer Faculty Research Program for sponsoring the travel to a Naval Research Facility and Dr. Alberto Piqué, Branch Head, Naval Research Laboratory, for granting him access to the microfabrication facilities in the Material Sciences and Technology Division. This research used resources of the Center for Functional Nanomaterials (CFN), which is a U.S. Department of Energy Office of Science User Facility, at Brookhaven National Laboratory under Contract No. DESC0012704.
2023-21245 | INT/EXT | Newsroom









