Controlling Oxide Catalysts with Peroxides
April 30, 2023
 enlarge
enlarge
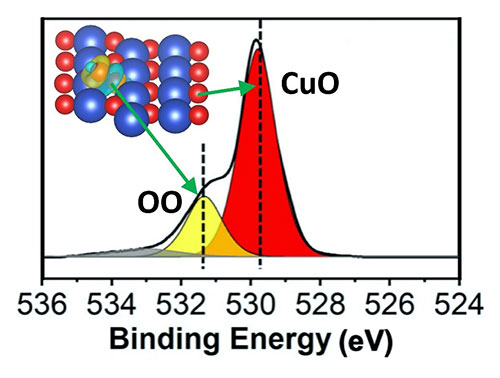
Peroxide formation on CuO by O2 annealing. Photoelectron spectrum of O 1s of the CuO. Inset: charge density difference distribution of the peroxide group formed by adsorbed O (yellow) at a site between Cu (blue) and its adjacent lattice O (red).
Scientific Achievement
CFN Users at Binghamton University and CFN staff demonstrated they could control the activity and selectivity of an oxide catalyst (cupric oxide, CuO) using adsorbed oxygen.
Significance and Impact
This work provides mechanistic insights into the effect of adsorbed oxygen on the tunability of redox steps for catalytic oxidation reactions, which can play a vital role in clean energy production and environmental remediation applications.
Research Details
Catalytic oxidation reactions cover a large part of the heterogeneous catalysis field, accounting for more than 60% of the chemicals and intermediates used in the chemical industry and playing a vital part in the remediation of hydrocarbon pollutants and the production of sustainable energy. The Mars-van Krevelen mechanism is the foundation for oxide catalyzed oxidation reactions and relies on spatiotemporally separated redox steps. This work demonstrated the tunability of this separation with peroxide species formed by excessively adsorbed oxygen, thereby modifying the catalytic activity and selectivity of the oxide. Using CuO as an example, we show that a surface layer of peroxide species acts as a promoter to significantly enhance CuO reducibility in favor of H2 oxidation but conversely as an inhibitor to suppress CuO reduction against CO oxidation. Together with atomistic modeling, it was identified that this opposite effect of the peroxide on the two oxidation reactions stems from its modification on coordinately unsaturated sites of the oxide surface. By differentiating the chemical functionality between lattice oxygen and peroxide, these results are closely relevant to a wide range of catalytic oxidation reactions using excessively adsorbed oxygen to activate lattice oxygen and tune the activity and selectivity of redox sites.
- The results demonstrate surface peroxide significantly enhances CuO reducibility in favor of H2 oxidation but suppresses CuO reduction against CO oxidation.
- Ambient pressure X-ray photoelectron spectroscopy (AP-XPS) and infrared reflection absorption spectroscopy (IRRAS) were used to determine the surface species in gas environments.
- The CFN Proximal Probes Facility and NSLS-II In situ and Operando Soft X-ray Spectroscopy (IOS) beamline were used for XPS and IRRAS characterization. CFN Theory & Computation was used for computational modeling.
CFN Capabilities: Ambient pressure X-ray photoelectron spectroscopy (AP-XPS) and infrared reflection absorption spectroscopy (IRRAS) from the CFN Proximal Probes Facility were used to determine the surface species in gas environments.
Publication Reference
Zhu Y.G., Wang J.Y., Patel S.B., Li C.R., Head A.R., Boscoboinik J.A., Zhou G.W. “Tuning the surface reactivity of oxides by peroxide species.” Proceedings of the National Academy of Sciences of the United States of America 120, 13 (2023).
DOI: https://doi.org/10.1073/pnas.2215189120
OSTI: www.osti.gov/biblio/1962691
Brookhaven Newsroom: Scientists Use Peroxide to Peer into Metal Oxide Reactions
Acknowledgment of Support
This work was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award No. DE-SC0001135. This research used the Proximal Probes Facility of the Center for Functional Nanomaterials, the 23-ID-2 (IOS) beamline at the National Synchrotron Light Source II, and the Scientific Data and Computing Center, a component of the Computational Science Initiative, which are U.S. DOE Office of Science Facilities, at Brookhaven National Laboratory under Contract No. DE-SC0012704.
2023-21265 | INT/EXT | Newsroom









