In Fuel Cell Catalysts, Three Elements are Better than One
August 30, 2023
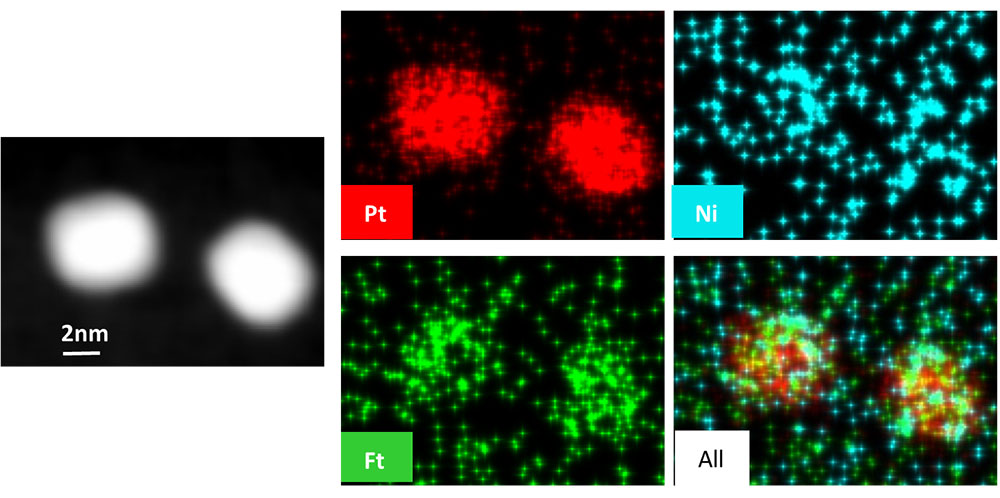
TEM image of two catalysts (left), elemental mapping showing catalyst compositions (right)broken out into Pt (red, top middle), Fe (green, lower middle), Ni (blue, top right), and then showing all three (lower right).
Scientific Achievement
CFN staff and users from Binghamton University co-led a study resulting in a new method to synthesize monodisperse, ternary (Pt-Fe-Ni) colloidal catalysts and measure their performance. These trimetallic materials showed superior mass (2.8x) and specific (5.6x) activities toward the oxygen reduction reaction (ORR) compared to the benchmark platinum catalyst, and retain performance after 10,000 voltage cycles in alkaline solution.
Significance and Impact
This synthetic strategy paves the way for improving reactivity for a broad range of electrocatalytic applications, including fuel cells and water electrolysis.
Research Details
The authors report a facile colloidal synthesis approach for the preparation of monodisperse Pt–Fe–Ni nanocrystals (NCs). The produced trimetallic catalyst supported on carbon(Pt–Fe–Ni NCs/C) exhibited superior mass and specific activities toward ORR in alkaline media. An accelerated durability test (ADT) indicated that the activity of Pt–Fe–Ni NCs/C was considerably retained after 10,000 potential cycles in 0.1 M KOH solution. Due to the incorporation of Fe and Ni atoms in Pt-lattice, this trimetallic electrocatalyst also manifests enhanced electrochemical performance toward oxygen evolution reaction (OER) with a smaller overpotential when compared with the Pt/C (?η = 0.20 V). The work showcases the feasibility of the Pt-based trimetallic NC synthesis and also demonstrates the effect of ORR and OER enhancement in alkaline media when 3D transition elements are introduced into the Pt lattice.
- Incorporation of Fe and Ni into the pt lattice also yields enhanced electrochemical activity and durability toward the OER with reduced overpotential when compared to the benchmark Pt/C catalyst.
CFN Capabilities: The CFN Electron Microscopy Facility was used to characterize the structure and composition of the nanocrystals.
Publication Reference
Li C., Pan J., Zhang L.* , Fang J.*, “Colloidal Synthesis of Monodisperse Trimetallic Pt-Fe-Ni Nanocrystals and Their Enhanced Electrochemical Performances.” Nanotechnology 34, 075401 (2023).
* Corresponding authors
DOI: 10.1088/1361-6528/aca337
https://iopscience.iop.org/article/10.1088/1361-6528/aca337
OSTI: https://www.osti.gov/biblio/1907836
Acknowledgment of Support
This work was primarily supported by the National Science Foundation under Grant # DMR 1808383. CL and JP were also partially supported by the Center for Alkaline-Based Energy Solutions (CABES), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under award # DESC0019445.
LZ acknowledges the use of TEM facilities for the structural characterization, at the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is supported by the U.S. DOE, Office of Science, Basic Energy Sciences under Contract No. DE-SC0012704. Partial low-magnification TEM imaging work was supported by S3IP/ADL, the State University of New York at Binghamton. We also appreciate the Harpur College Faculty Research Grant/Subvention Fund for supporting the color figure print.
2023-21456 | INT/EXT | Newsroom









