Unveiling Dynamics of Pt/CeO2 Catalysts in H2 production through the Water-Gas-Shift Reaction
January 21, 2025
Scientific Achievement
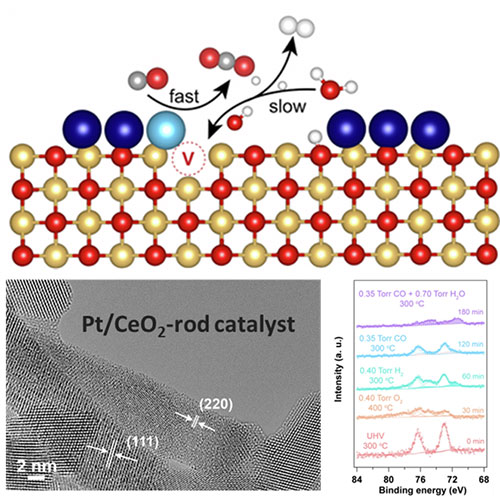
Using in situ spectroscopy and microscopy tools, CFN and NSLS-II researchers mapped the dynamic restructuring of Pt/CeO2 active sites during the water-gas-shift reaction, identifying transformation pathways critical to catalytic efficiency.
Significance and Impact
The insights could guide the design of highly efficient and stable catalysts for hydrogen production and other energy-related applications.
Research Details
With increasing energy demands, the production of clean and affordable energy is a critical challenge, and hydrogen (H2) is a promising energy carrier. Currently, most hydrogen is produced from steam reforming of fossil fuels, which produces carbon monoxide (CO) as a byproduct. The water-gas shift reaction (WGSR) is necessary to remove CO and produce high-purity hydrogen for various applications. This research investigates the dynamic nature of active sites in Pt/CeO2 catalysts during the water-gas-shift reaction (WGSR) using multiple in situ characterization techniques. The study reveals that the catalytic performance of the catalysts is directly correlated with the concentration of interfacial Ptδ+ –O –Ce4+ moieties, with a performance order of Pt/CeO2-rod > Pt/CeO2-cube > Pt/CeO2-oct. During the WGSR, metallic Pt (Pt0) forms, which leads to the transformation of active sites to Pt0 –Ov –Ce3+ and a reconstruction of the interface. The in situ techniques, including environmental transmission electron microscopy (ETEM) and ambient pressure X-ray photoelectron spectroscopy (AP-XPS), show that surface atoms are highly mobile, and small nanoclusters are formed on the surface during the reaction. These findings highlight the dynamic nature of the active sites at the Pt/CeO2 interface and their influence on catalytic activity during the WGSR.
- Combined environmental TEM, IR (CFN), and AP-XPS (NSLS-II) to study how Pt and CeO2 interfaces evolve, using different nano-shapes of CeO2 supports.
- The work linked higher catalytic activity to forming specific Pt0-Ov-Ce3+ (oxygen vacancies) sites under reaction conditions.
- The active sites are more likely to form when the CeO2 has a nanorod shape.
Materials Synthesis was done at the CFN. In situ characterization during the water-gas-shift reaction was done using Environmental Transmission Electron Microscopy (ETEM) and transmission infrared (TIR) at the Proximal Probes facilities at the CFN and Ambient Pressure X-ray Photoelectron Spectroscopy (AP-XPS) at NSLS-II.
Publication Reference
Gengnan Li, Dmitri N. Zakharov, Tianhao Hu, Youngseok Yu, Iradwikanari Waluyo, Adrian Hunt, Ashley R. Head, Jorge Anibal Boscoboinik. Tracking the dynamics of catalytic Pt/CeO2 active sites during water-gas-shift reaction. Communications Materials, 5, 133, (2024).
DOI: https://doi.org/10.1038/s43246-024-00575-4
OSTI: https://www.osti.gov/pages/biblio/2406093
Acknowledgment of Support
This work was supported by the U.S. Department of Energy, Office of Basic Energy Science, under Award DE-SC0022199. AP-XPS experiments were carried out at the 23-ID-2 (IOS) beamline of the National Synchrotron Light Source II, Brookhaven National Laboratory, supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-SC0012704. Catalysts synthesis, characterization and performance tests were conducted using the Proximal Probes, Electron Microscopy, and Materials and Characterization Facilities at the Center for Functional Nanomaterials, supported by U.S. Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-SC0012704. The authors are thankful to Dr. Dmytro Nykypanchuk for his assistance in X-ray Diffraction measurement.
2025-22304 | INT/EXT | Newsroom