Transforming the Composition of Nanoparticles Using Electrochemistry
December 10, 2014
What Is The Scientific Achievement?
 enlarge
enlarge
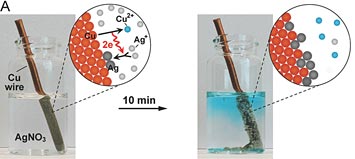
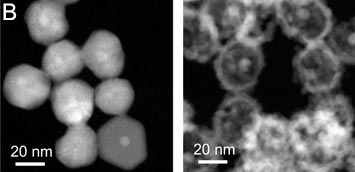
A: Images of a copper wire undergoing galvanic replacement when immersed in a silver-based solution. The associated cartoons illustrate the replacement mechanism for copper and silver, resulting in silver coating of the wire. B: Electron microscopy images of the galvanic replacement reaction, transforming a silver nanoparticle into a hollow silver/palladium nanostructure.
Galvanic replacement reactions provide an elegant way of transforming solid nanoparticles into complex hollow morphologies. Conventionally, galvanic replacement is studied by stopping the reaction at different stages and characterizing the products ex situ. In situ observations by liquid-cell electron microscopy can provide insight into mechanisms, rates and possible modifications of galvanic replacement reactions in the native solution environment. Here, we use liquid-cell electron microscopy to investigate galvanic replacement reactions between silver nanoparticle templates and aqueous palladium salt solutions. Our in-situ observations follow the transformation of the silver nanoparticles into hollow silver–palladium nanostructures. While the silver–palladium nanocages have morphologies similar to those obtained in ex-situ control experiments the reaction rates are much higher, indicating that the electron beam strongly affects the galvanic-type process in the liquid-cell. By using scavengers added to the aqueous solution we identify the role of radicals generated via radiolysis by high-energy electrons in modifying galvanic reactions.
 enlarge
enlarge
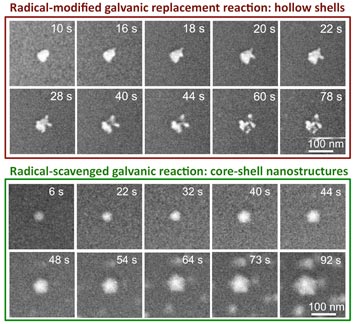
Top: Sequence of STEM images that follow the real-time evolution of a silver nanoparticle into a hollow silver/palladium structure wile in a palladium-based solution. Bottom: Sequence of STEM images that follow the real-time evolution of a silver nanoparticle into a hollow silver/palladium structure wile in a palladium-based solution with added isopropanol. In this case, the silver particle does not become entirely hollowed out. In fact, the reaction shifts toward growth, and a Ag core-Pd shell nanostructure is formed.
Why Does This Matter?
Understanding how to control galvanic replacement reactions in nanoparticles is important for exploiting these materials in applications, such as nanoscale containers for diagnostics and drug delivery, contrast enhancement agents in biomedical imaging, or platforms for facilitating chemical reactions.
What Are The Details?
- CFN Capabilities: CFN’s Electron Microscopy facility provided an environmental transmission electron microscope with a special holder that can accept the liquid cell.
Publication Reference
In situ liquid-cell electron microscopy of silver–palladium galvanic replacement reactions on silver nanoparticles
E. Sutter1, K. Jungjohann1, S. Bliznakov2, A. Courty3, E. Maisonhaute4, S. Tenney1, and P. Sutter1
1. Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton, New York 11973, USA
2. Department of Chemistry, Brookhaven National Laboratory, Upton, New York 11973, USA
3. Sorbonne Universités, UPMC Univ Paris 06, UMR 8233, CNRS, Laboratoire Monaris, Paris F-75005, France
4. Sorbonne Universités, UPMC Univ Paris 06, UMR 8235, Laboratoire Interfaces et Systèmes Electrochimiques, Paris F-75005, France
Nature Communications 5, 4946 (2014).
Acknowledgment of Support
This research has been carried out at the Center for Functional Nanomaterials, the Brookhaven National Laboratory, which is supported by the US Department of Energy, the Office of Basic Energy Sciences, under Contract No. DE-AC02–98CH10886. This work was supported in part (AC and EM) by the LabEx MiChem part of French state funds managed by the ANR within the Investissements d'Avenir programme under reference ANR-11-IDEX-0004-02.
2014-5414 | INT/EXT | Newsroom