Swapping Tellurium for Sulfur Improves Light Absorption in Organic Solar Cells
February 5, 2015
What Is The Scientific Achievement?
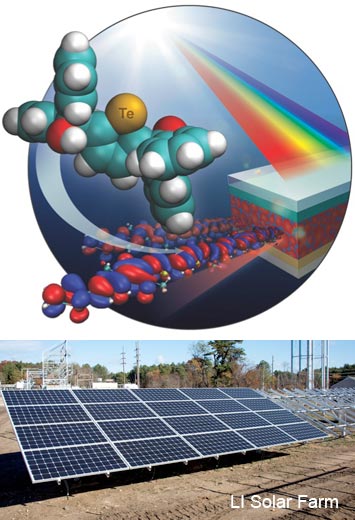
Tellurium-containing polymer-based solar cells can absorb light across an exceptionally broad spectrum and have the potential to enhance power conversion efficiencies.
The investigation of light absorbing organic semiconductors is important for the development of lightweight flexible solar cells. Replacing sulfur atoms in commonly used, polymer-based solar cells with tellurium atoms results in materials that absorb a wider range of wavelengths of sunlight. A tellurophene-containing low-bandgap polymer (PDPPTe2T) was synthesized by microwave-assisted palladium-catalyzed ipso-arylative polymerization of 2,5-bis[(α-hydroxy-α,α-diphenyl)methyl]tellurophene with a diketopyrrolopyrrole (DPP) monomer. This work has demonstrated that solar cells constructed from such polymers can convert sunlight into electrical current with an efficiency of 4.4% at wavelengths up to 1.0 microns. This result is a benchmark for tellurium-based polymer solar cells. Density functional theory calculations (DFT) suggest that the switch from sulfur to tellurium shifts the absorption spectrum toward longer wavelengths in the solar spectrum.
Why Does This Matter?
These are the first reported solar cells comprised of such tellurium-based polymers. As well, these polymers absorb light across a broad range of wavelengths from ultraviolet to infrared, generating electricity at> 4% efficiency.
What Are The Details?
- CFN Capabilities: CFN’s Materials Synthesis & Characterization and Theory & Computation Facilities were used for polymer synthesis, device preparation, electrical characterization, and computation assistance.
Publication Reference
Polymerization of Tellurophene Derivatives by Microwave-Assisted Palladium-Catalyzed ipso-Arylative Polymerization
Dr. Young S. Park1, Dr. Qin Wu1, Dr. Chang-Yong Nam1 and Prof. Robert B. Grubbs1,2
1 Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton, NY 11973 (USA)
2 Department of Chemistry, Stony Brook University, Stony Brook, NY 11794 (USA)
Angewandte Chemie International Edition 53 10691-10695 (2014).
Acknowledgment of Support
This research was carried out at the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-AC-02-98CH10886. This research was also supported by the BNL Laboratory Directed Research and Development Award 09-003. Mass spectrometry was performed at the Proteomic Center, Stony Brook University, shared instrumentation grant: NIH/NCRR 1 S10 RR023680-1.
2015-5527 | INT/EXT | Newsroom









