Metal Oxidation – Controlled by Atomic Surface Steps
March 18, 2015
What Is The Scientific Achievement?
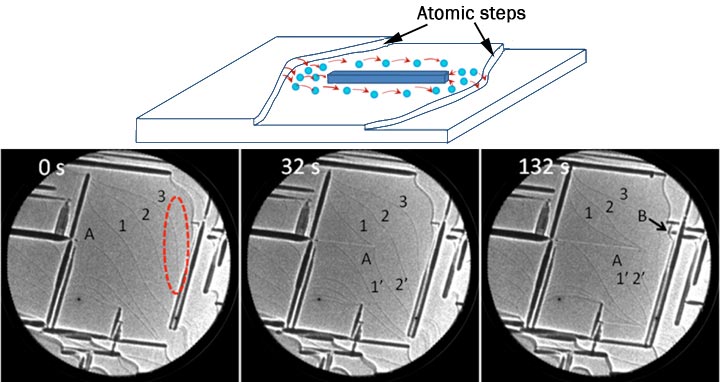
Rust never sleeps. Whether a reference to the 1979 Neil Young album or a product designed to protect metal surfaces, the phrase invokes the idea that corrosion from oxidation—the more general chemical name for rust and other reactions of metal with oxygen—is an inevitable, persistent process. But a new study performed at the Center for Functional Nanomaterials (CFN) at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory reveals that certain features of metal surfaces can stop the process of oxidation in its tracks. The findings, published in the Proceedings of the National Academy of Sciences, could be relevant to understanding and perhaps controlling oxidation in a wide range of materials—from catalysts to the superalloys used in jet engine turbines and the oxides in microelectronics. The team used a low-energy electron microscope (LEEM) to capture changes in the surface structure of a nickel-aluminum alloy as “stripes” of metal oxide formed and grew under a range of elevated temperatures. The metal Zhou wanted to study, nickel-aluminum, has a characteristic common to all crystal surfaces: a stepped structure composed of a series of flat terraces at different heights. The steps between terraces are only one atom high, but they can have a significant effect on material properties. Being able to see the steps and how they change is essential to understanding how the surface will behave in different environments, in this case in response to oxygen.
 enlarge
enlarge
(top) Schematic of the surface of a Ni-Al alloy with atomic-height surface steps, present on all crystalline materials. Aluminum oxide (Al2O3) grows by incorporating aluminum atoms released from the steps. As the oxide encounters a step, its further growth becomes inhibited. (bottom) In-situ microscopy image sequence illustrating the encounter of an Al2O3 stripe ('A') with surface steps (faint black lines).
Why Does This Matter?
While the formation of thicker oxide layers has been studied extensively, the initial stages of surface oxidation remain poorly understood, particularly for complex materials such as metal alloys. The findings of this study demonstrate that atomic-height steps can play a key role in limiting the onset of oxidation. This points to new avenues for controlling metal oxidation in applications ranging from catalysis to corrosion protection and microelectronics.
What Are The Details?
- CFN Capabilities: The unique in-situ surface microscopy capabilities in the CFN’s Proximal Probes Facility were instrumental in enabling the direct observation of the interaction of oxide stripes with surface steps on NiAl(100). Microscopy with combined high spatial and temporal resolution allowed measuring in oxygen gas atmospheres how the rate of oxidation is reduced when the growing oxide encounters atomic-height steps on the surface.
Publication Reference
Oxidation-driven surface dynamics on NiAl(100)
Hailang Qin1, Xidong Chen2, Liang Li1, Peter Sutter3, and Guangwen Zhou1
1Department of Mechanical Engineering & Multidisciplinary Program in Materials Science and Engineering, State University of New York, Binghamton, New York 13902, USA.
2Department of Chemistry, Physics and Engineering, Biola University, La Mirada, CA 90639.
3Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton, NY 11973, USA
Proc. Nat. Acad. Sci. USA 112, E103-E109 (2015).
Acknowledgment of Support
This work was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award No. DE-FG02-09ER46600. Research carried out in part at the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886. This work used the computational resources from the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation grant number OCI-1053575.
2015-5594 | INT/EXT | Newsroom









