Watching Ionic Liquids at Electrode Interfaces in Real Time
June 26, 2017
What is the scientific achievement?
Ionic liquids — liquid salts made by combining positively charged cations and negatively-charged anions — have potential uses as advanced battery electrolytes. When the electrolytes contact an electrode, they form a 'double-layer' a few nm thick, where important electrochemical reactions take place. CFN scientists have created a technique to observe in real time how ions in the double-layer reconfigure under applied voltage bias.
Why does this achievement matter?
Determining the electrolyte response to applied voltages is key to optimizing the performance of ionic liquids for energy storage devices.
What are the details?
 enlarge
enlarge
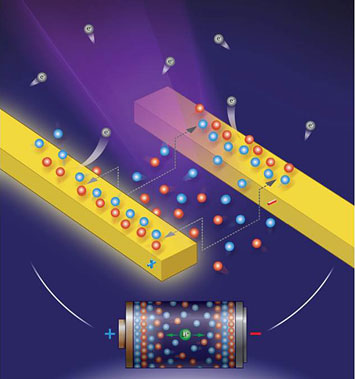
An illustration of the experimental setup for tracking real-time evolution of ionic liquid: an ultraviolet light shines on biased electrodes covered with iconic liquid to excite photoelectrons, which are collected to form a spatially resolved image.
CFN Capabilities:
The CFN aberration-corrected low-energy electron / photoemission electron microscope was used to visualized the ionic liquid under voltage bias.
Publication Reference
W. Sitaputra, D. Stacchiola, J. F. Wishart, F. Wang, J. T. Sadowski, In Situ Probing of Ion Ordering at an Electrified Ionic Liquid/Au Interface, Advanced Materials 10.1002/adma.201606357 (2017).
Semiconductor Engineering News Release
Acknowledgement of Support
This work was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences, under contract # DE-SC0012704, and it used resources of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility at Brookhaven National Laboratory. F.W. was supported by the Laboratory Directed Research and Development (LDRD) program at Brookhaven National Laboratory.
2017-12324 | INT/EXT | Newsroom









