Nanocrystals Help Magnesium Batteries Go On-the-Move
October 26, 2018
The CFN User and Staff Highlight is research from the Toyota Research Institute of North America and Michigan State University’s Center for Advanced Microscopy.
 enlarge
enlarge
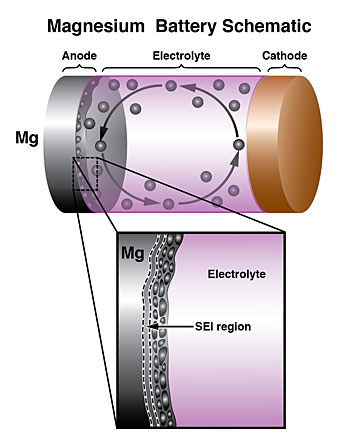
(Top) Schematic of a rechargeable battery with magnesium (Mg) anode. (Bottom) Close-up of the Mg anode/electrolyte interface, showing the solid electrolyte interphase and formation of Mg nanocrytals during battery operation.
What is the scientific achievement?
CFN users from the Toyota Research Institute of North America and Michigan State University, working with CFN staff, measured the operational mechanism of rechargeable batteries with magnesium (Mg) metal anodes. Operando scanning transmission electron microscopy revealed that the combination of a highly functional solid electrolyte interphase at the anode, and Mg nanocrystals formed during operation, is key to improved performance.
Why does this achievement matter?
Magnesium has five times more volumetric energy storage density compared to graphite anodes used in commercial lithium-ion batteries. The reported unique operating mechanism enables high battery charging/discharging rates (10 mA·cm-2) at low temperatures (0 °C), which are important for transportation applications.
What are the details?
Morphological control of electrochemically deposited metallic anodes, such as Li, Zn, and Mg, under high applied charging/discharging rates is essential for development of high-energy-density batteries. For transportation applications, maximizing applied rates and energy density is key to attaining viable customer acceleration and range expectations, respectively. In this work, we observe and characterize the in-situ generation of Mg nanocrystals during battery operation, which facilitates battery cycling under high rates (10 mA cm–2) at low temperature (0 °C). Through operando scanning transmission electron microscopy analysis, we discovered a highly functional solid-electrolyte interphase (SEI), a first of its kind, which enabled continuous deposition and dissolution of Mg without internal shorting. The unique morphology of the deposited Mg and the functional capability of the SEI are key to future development of practical metallic Mg anodes. In this work, we monitored the nucleation and growth of Mg nanocrystals in real-time, which was key to understanding the mechanism by which the observed Mg morphologies form during battery operation.
CFN Capabilities:
The CFN Electron Microscopy Facility was used to conduct in-situ and operando characterization.
Publication Reference
N. Singh, T. S. Arthur, O. Tutusaus, J. Li, K. Kisslinger, H. L. Xin, E. A. Stach, X. Fan, R. Mohtadi, Achieving High Cycling Rates via In Situ Generation of Active Nanocomposite Metal Anodes, ACS Applied Energy Materials 1, 4651 (2018).
Acknowledgement of Support
This research used resources of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DESC0012704.
2018-14399 | INT/EXT | Newsroom









