Observing the Obstacles Underlying Battery Capacity Fade
June 28, 2019
What is the scientific achievement?
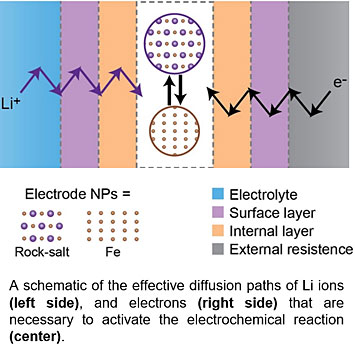
CFN staff and collaborators studied the performance of magnetite (Fe3O4) as an inexpensive, nontoxic battery material. Batteries made using magnetite can have high capacities, but unfortunately their capacity fades with battery cycling. The team combined in-situ transmission electron microscopy and synchrotron X-ray absorption spectroscopy to understand the origins of this capacity —directly observing the accumulation of obstacles to electron transport in the magnetite material.
Why does this achievement matter?
In-situ transmission electron microscopy allowed direct observations of electrode structural changes in real time. Understanding how kinetic barriers are linked to capacity fading in materials is important for their future practical implementation.
What are the details?
Batteries with conversion-type electrodes exhibit higher energy storage density but suffer much more severe capacity fading than those with conventional intercalation-type electrodes. This capacity fading has been considered to result from contact failure between the active material and the current collector, or the breakdown of solid electrolyte phase layer. In this work, we focused on the phase evolution occurring in iron oxide at later-stage cycles to investigate the capacity loss issues. Using a combination of synchrotron X-ray absorption spectroscopy and in-situ transmission electron microscopy, we found that the conversion reaction occurring after several cycles is completely different from the initial one. Furthermore, the reverse-conversion reaction is not fully reversible. Thus, residues of Li2O and Fe, which are products of the conversion reaction, are found at charged state and the portion of Li2O and Fe becomes higher as the electrochemical cycle proceeds. The accumulation of an electronic-insulating internal passivation phase and the surface layer over cycling enforce a rate-limiting diffusion barrier for electron transport, which is responsible for the capacity degradation and poor rate capability. This work directly links the performance with the microscopic phase evolution in cycled electrode materials and provides insights into designing conversion-type electrode materials for applications.
CFN Capabilities
The CFN Electron Microscopy Facility was used for in-situ characterization.
Publication Reference
J. Li, S. Hwang, F. Guo, S. Li, Z. Chen, R. Kou, K. Sun, C.-J. Sun, H. Gan, A. Yu, E. A. Stach, H. Zhou, D. Su, Phase evolution of conversion-type electrode for lithium ion batteries, Nature Communications 10, 2224 (2019).
https://doi.org/10.1038/s41467-019-09931-2
DOI: 10.1038/s41467-019-09931-2
https://www.bnl.gov/newsroom/news.php?a=115515
https://www.osti.gov/biblio/1542127-phase-evolution-conversion-type-electrode-lithium-ion-batteries
Acknowledgement of Support
This work is supported by the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357 and the Canadian Light Source and its funding partners. The authors also acknowledge the support provided by the Natural Sciences and Engineering Research Council of Canada (NSERC), University of Waterloo, and Waterloo Institute for Nanotechnology.
2019-16704 | INT/EXT | Newsroom