Nanocomposite Liposomes Kill Cancer Cells
June 29, 2020
 enlarge
enlarge
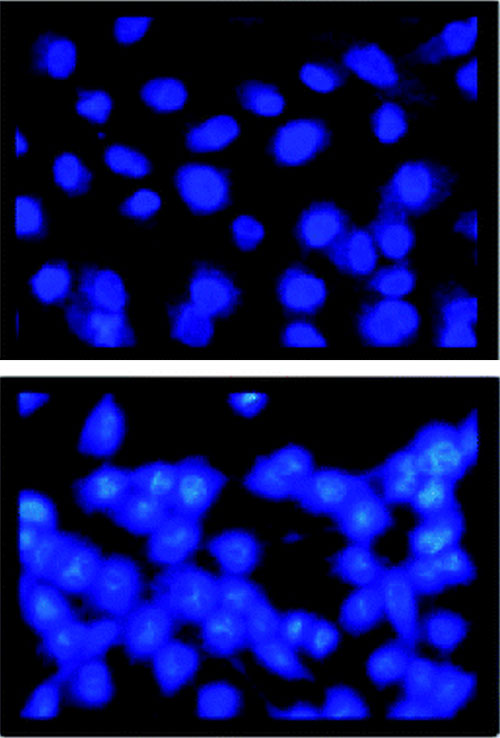
Optical microscopy images of cancer cells treated with the nanocomposite liposome medicine in pH 7.4 (top) and pH 5.4 (bottom); cell death occurred at pH 5.4 but not pH 7.4.
What is the scientific achievement?
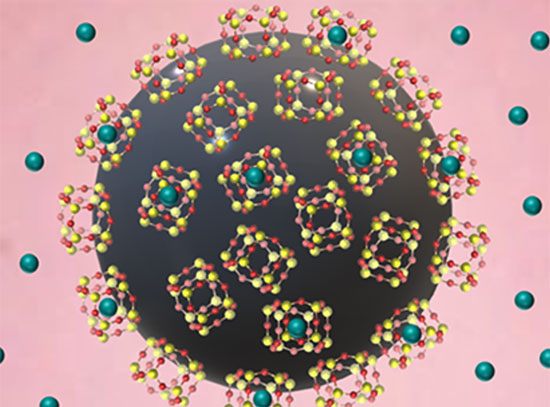
CFN users from SUNY Old Westbury developed a nanocomposite cancer medicine by attaching a porphyrin drug to silica and encapsulating it within a nanocomposite liposome. The liposome encapsulant limits drug release to tumor cells, which have an acidic pH, avoiding healthy cells, which are more pH-neutral. The team also showed that singlet oxygen production—used to kill tumor cells—is quintupled in acidic pH compared to neutral pH.
Why does this achievement matter?
Made from phospholipids and biocompatible with cell membranes, liposomes are promising vehicles for nutrient and drug delivery. Lipid selection and modification can be useful for tuning drug targets.
What are the details?
It is both challenging and desirable to have drug sensitizers released at acidic tumor pH for photodynamic therapy in cancer treatment. A pH-responsive carrier was prepared, in which fumed silica-attached 5,10,15,20-tetrakis(4-trimethylammoniophenyl)porphyrin (TTMAPP) was encapsulated in 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) nanocomposite liposomes. The sizes of agglomerates were determined by dynamic light scattering to be 115 nm for silica and 295 nm for silica-TTMAPP-DOPC liposomes. Morphological changes captured via transmission electron microscopy (TEM) showed liposome formation at pH 8.5 and collapse upon silanol protonation. TTMAPP release was enhanced from 13% at pH 7.5 to 80% at pH 2.3, as determined spectrophotometrically through dialysis membranes. The fluorescence emission of TTMAPP encapsulated in the dry film of liposomes was reduced to half at pH 8.6 when compared to the emission at pH 5.4; the production of singlet oxygen was quintupled at pH 5.0 compared to pH 7.6. For human prostate cancer cells treated with liposomes containing 6.7 µM, 13 µM, 17 µM, and 20 µM TTMAPP, the cell viabilities were 60%, 18%, 20%, and 5%, respectively at pH 5.4; 58%, 30%, 25%, and 10%, respectively, at pH 6.3; and 90%, 82%, 68%, and 35%, respectively at pH 7.4. Light-induced apoptosis in cancerous cells was only observed in the presence of liposomes at pH 6.3 and pH 5.4, as indicated by chromatin condensation.
CFN Capabilities
CFN Advanced Optical Microscopy & Spectroscopy, Materials Synthesis & Characterization, and Electron Microscopy facilities were used to characterize the nanocomposite drug carrier.
Publication Reference
G.V. Fuentes, E.N. Doucet, A. Abraham, N.K. Rodgers, F. Alonso, N. Euceda, M.H. Quinones, P.A. Riascos, K. Pierre, N.H. Sarker, M. Dhar-Mascareno, M. Cotlet, K. Kisslinger, F. Camino, M. Li, F. Lu and R. Gao, Nanocomposite liposomes for pH-controlled porphyrin release into human prostate cancer cells, RSC Advances 10, 17094 (2020).
DOI: 10.1039/d0ra00846j
Acknowledgement of Support
We thank the support from Chemistry and Physics Department, Biological Sciences Department, Faculty Development Grants at SUNY Old Westbury. This research used resources of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. The work is also partially supported by NSF-IUSE (1611887), NIH (R15CA169984), NYSED-CSTEP (C402579), NYSED-STEP (C402605) and NYS DOH01-C30319GG at SUNY Old Westbury. Authors also thank Professor Judith Lloyd at SUNY Old Westbury for helpful discussions.
2020-17418 | INT/EXT | Newsroom