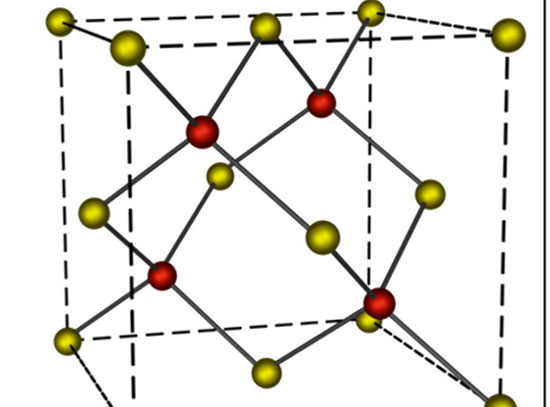
Peering at Dynamic Perimeters that Promote Catalytic Activity
August 31, 2021
 enlarge
enlarge
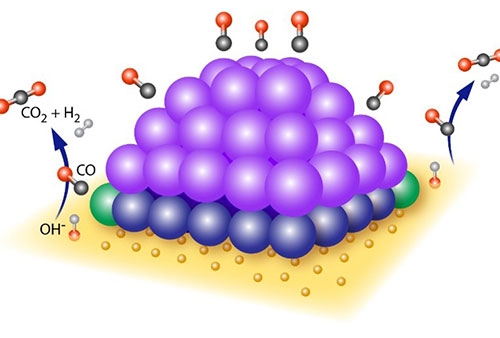
Schematic of the active platinum (Pt)/ceria structure at high temperature. Purple spheres: metallic Pt atoms; blue spheres: oxidized (2+) Pt atoms; green spheres: active perimeter Pt sites.
What is the scientific achievement?
CFN users and staff experimentally determined the mechanism by which ceria-supported platinum (Pt) catalysts facilitate the water-gas-shift reaction. Multimodal in situ and ex situ characterization under working conditions illuminate the dynamic changes driving catalytic activity. These measurements showed that the reaction is catalyzed at the perimeter of the Pt-ceria interface at high temperature, with missing oxygen atoms in the ceria also involved in forming the dynamic active site.
Why does this achievement matter?
Oxide-supported precious metal catalysts are of interest for the industrially important water-gas shift reaction to produce hydrogen because of their low-temperature activity, selectivity, and stability. Knowing which sites enable the reaction to proceed can inform the design of catalysts with higher activity at lower cost.
What are the details?
Oxide-supported noble metal catalysts have been extensively studied for decades for the water-gas shift (WGS) reaction. This reaction, in which carbon monoxide and water are transformed into carbon dioxide and hydrogen gas, is an important step in producing and purifying hydrogen for various applications—including as a clean fuel in fuel-cell vehicles and to synthesize hydrocarbons and ammonia. Among WGS reaction catalysts, ceria-supported noble metal (platinum and gold) catalysts have recently been heavily investigated. However, the identity of the active species/sites of these catalysts has been widely debated. There remains considerable uncertainty as to how the specific features of the active-metal-support interfacial bonding—perhaps most importantly, the temporal dynamic changes occurring therein—serve to enable high activity and selectivity. An understanding of all parts of this complex interacting system (metal catalyst, support, adsorbates) at the atomic level is difficult to obtain without correlative, multimodal, in situ/operando modes of characterization.
The work combines multiple advanced microscopy and spectroscopy techniques available at Brookhaven and Argonne National Laboratories to reveal the dynamic nature of active platinum (Pt) sites in ceria-supported Pt (Pt/CeO2) nanoclusters at the atomic level. In situ infrared spectroscopy identified the active Pt species, while combined in situ transmission electron microscopy (TEM), diffuse reflectance infrared Fourier-transform spectroscopy (DRIFTS), ambient-pressure x-ray photoelectron spectroscopy (AP-XPS), and x-ray absorption spectroscopy (XAS) revealed the nature of the active site: Pt0−O vacancy−Ce3+. Our results reveal the synergistic effects of metal-support bonding at the perimeter region. We find that the perimeter Pt0−O vacancy−Ce3+ sites formed in the active structure are transformed at working temperatures and regulate the adsorbate behaviors. The dynamic nature of these perimeter sites is a key mechanistic step for the WGS reaction. Understanding how these dynamic changes are connected to reactivity will inform the targeted design of higher-activity catalysts that use less of the expensive precious metals.
CFN Capabilities
The CFN Electron Microscopy Facility was used for in situ transmission electron microscopy.
Publication Reference
Y. Li, M. Kottwitz, J.L. Vincent, M.J. Enright, Z. Liu, L. Zhang, J. Huang, S.D. Senanayake, W-C.D. Yang, P.A. Crozier, R.G. Nuzzo, and A.I. Frenkel. “Dynamic structure of active sites in ceria-supported Pt catalysts for the water gas shift reaction,” Nature Communications 12, 914 (2021).
DOI: https://doi.org/10.1038/s41467-021-21132-4
OSTI: https://www.osti.gov/biblio/1766166-dynamic-structure-active-sites-ceria-supported-pt-catalysts-water-gas-shift-reaction
Brookhaven Lab news release: “Study Reveals Platinum’s Role in Clean Fuel Conversion”
Acknowledgement of Support
Y.L., A.I.F., and R.G.N. acknowledge support for this work by the U.S. Department of Energy, Office of Basic Energy Sciences under Grant No. DE-FG02-03ER15476. Reaction tests and DRIFTS measurements at Brookhaven National Laboratory’s Chemistry Division and XAFS data analysis were made possible due to the Program Development fund 21-017 to A.I.F. M.K. and R.G.N. acknowledge fellowship support from the Department of Chemistry at the University of Illinois. Work by S.D.S. and Z.L. at Brookhaven National Laboratory was supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences under contract no. DE-SC0012704. S.D.S. is partially supported by a US Department of Energy Early Career Award. This research used Hitachi2700C STEM of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. The XAFS experiments used resources of the Advanced Photon Source Beamline 20ID at Argonne National Laboratory, which is an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science and was supported by the U.S. DOE under contract no. DE-AC02-06CH11357. J.L.V. and P.A.C. gratefully acknowledge NSF grant CBET-1604971 and the facilities at the National Institute of Standards and Technology at Gaithersburg, M.D. as well as those at the John M. Cowley Center for High Resolution Electron Microscopy at Arizona State University. W.D.Y. acknowledges support under the Cooperative Research Agreement between the University of Maryland and the National Institute of Standards and Technology Physical Measurement Laboratory, award 70NANB14H209, through the University of Maryland.
2021-19111 | INT/EXT | Newsroom