Solar-Powered Chlorine Production: A Sea Change in Water Splitting
April 30, 2024
 enlarge
enlarge
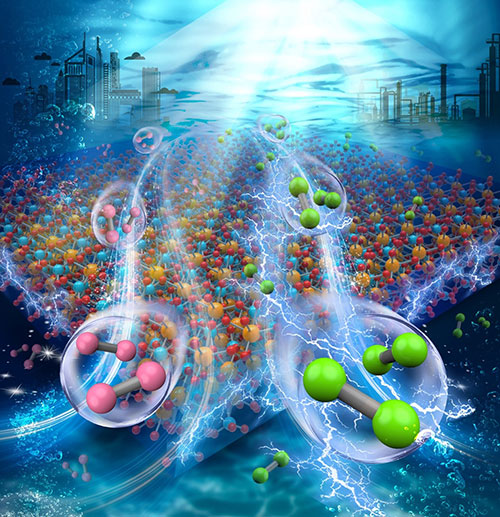
Chlorine (green) is produced simultaneously with hydrogen (red) through solar water splitting in brine, over a metal oxide photoanode.
Scientific Achievement
CFN researchers demonstrated a method to generate chlorine and hydrogen from seawater by solar water splitting, using bismuth vanadate and a cobalt oxide co-catalyst. The approach bypasses the bottleneck of the inefficient oxygen evolution reaction.
Significance and Impact
This approach has potential to reduce the energy footprint of the chloralkali industry and repurpose oceanic chlorine for clean energy production, while also providing clean hydrogen fuel.
Research Details
Solar water splitting promises an immensely attractive energy future, in which water, one of the most abundant resources on earth, is turned into hydrogen (H2), a clean fuel with high energy density. However, as demonstrated by research over the past half century, solar water splitting over semiconductor photoelectrodes poses a variety of complex materials science challenges, including the large overpotential required for the oxygen (O2) evolution reaction (OER). Although the overpotential may be partially reduced with an OER co-catalyst, in the end, all the efforts devoted to OER lead to a product (O2) that has negligible economic value on planet Earth. In water splitting, OER is a necessity only because an electron donor is needed for water reduction into H2, and water is the most readily available electron donor. One can avoid OER if another earth abundant electron donor is identified.
One particularly attractive candidate is the chloride ion (Cl-), which has a concentration of about 0.6 M throughout the oceans and thus constitutes a virtually unlimited supply. The chlorine evolution reaction (2Cl-→Cl2+2e-, ClER) follows a simpler reaction pathway than OER and has much lower overpotential. As an important chemical industry product and feedstock, chlorine carries much more significant economic value than oxygen. The industrial production of chlorine typically relies on the energy-intensive chloralkali process that accounts for approximately 1% of global electricity consumption. Enabling photoelectrochemical chlorine generation (PEC-Cl) will enable a very significant energy benefit.
This work reports the effective PEC-Cl from a brine solution, over epitaxial, crystalline bismuth vanadate (BiVO4) thin film photoanodes coated with a cobalt oxide (CoOx) co-catalyst, which is selected for its earth abundance, outstanding stability in both alkali and acid conditions, and activity as an oxidative electrocatalyst. The study finds that the activity of the chlorine generation reaction (ClER) is optimized when the thickness of CoOx is about 3 nm, with the faradic efficiency of ClER exceeding 60%.Detailed studies show that the CoOx cocatalyst layer is amorphous, uniform in thickness, and chemically robust. As such, the cocatalyst also effectively protects the underlying BiVO4 photoanodes against chlorine corrosion. This work provides insights into using solar water splitting for by-products that carry significant economic value while avoiding the energetically expensive oxygen evolution reactions. Optimal 3nm CoOx thickness yields a faradic efficiency for chlorine generation >60%. The amorphous CoOx acts as a protective layer against corrosion.
CFN Materials Synthesis, Electron Microscopy, & Proximal Probes facilities were used.
Publication Reference
Z. Y. Xi, C. Y. Zhou, K. Kisslinger, T. Nanayakkara, F. Lu, X. Tong, M. Z. Liu. “Cobalt Oxide-Coated Single Crystalline Bismuth Vanadate Photoanodes for Efficient Photoelectrochemical Chlorine Generation.” ACS Applied Materials & Interfaces 15, 42 (2023).
OSTI: www.osti.gov/biblio/2222385
Acknowledgment of Support
This research used Materials Synthesis & Characterization, Electron Microscopy, and Proximal Probes facilities of Center for Functional Nanomaterials (CFN), which is a U.S. Department of Energy Office of Science User Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704.
2024-21897 | INT/EXT | Newsroom









