Electron Microscopy Reveals in-Situ Evolution of Lithium Batteries
July 1, 2016
What Is The Scientific Achievement?
Magnetite (Fe3O4), a promising candidate as an anode in lithium ion batteries, is relatively inexpensive and non-toxic. However, the phase transitions that it experiences while operating within a battery remain poorly understood due to the kinetics of lithium ions and local electrochemical inhomogeneities. Using scanning transmission electron microscopy (STEM) techniques that are sensitive to strain, the intercalation of lithium into Fe3O4 nanoparticles (NPs) and the subsequent conversion reactions in the material were investigated in real time. Lithium intercalation processes were found to overlap significantly with subsequent conversion reactions within single NPs, which led to the coexistence of three distinct charging pathways within the NPs. This phenomenon has not been previously reported.
Why Does This Matter?
Intercalation reactions in Fe3O4 may determine the performance of lithium-ion battery materials undergoing lithiation; however, such reactions are difficult to probe in real time. This study utilized advanced in-situ electron microscopy techniques to identify and to investigate kinetically-driven phase evolution within Fe3O4 NPs.
What Are The Details?
 enlarge
enlarge
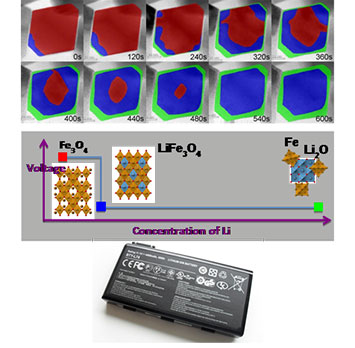
Top) STEM image series, overlaid false colors showing phase evolution upon lithiation, pristine Fe3O4 (red), Li-inserted LixFe3O4 (blue), and Fe+Li2O composite after conversion (green). Middle) The reaction route revealed in this work, from magnetite to rock-salt, then to nanocomposites of Fe and Li2O. Bottom) This work could have an impact on understanding the kinetics (and subsequent stability and longevity) of batteries while they are operating.
CFN Capabilities: CFN's Electron Microscopy Facility's JEOL-2100F and HD2700C Electron Microscopes were used to characterize the samples.
Publication Reference
Visualizing Non-Equilibrium Lithiation of Spinel Oxide via In Situ Transmission Electron Microscopy
Kai He1,*, Sen Zhang2,*, Jing Li1, Xiqian Yu1, Qingping Meng1, Yizhou Zhu3, Enyuan Hu1, Ke Sun1, Hongseok Yun2, Xiao-Qing Yang1, Yimei Zhu1, Hong Gan1, Yifei Mo3, Eric A. Stach1, Christopher B. Murray2, and Dong Su1
1Brookhaven National Laboratory, Upton, New York 11973, USA.
2Department of Chemistry, University of Pennsylvania, Philadelphia, Pennsylvania 19104, USA.
3Department of Materials Science and Engineering, University of Maryland, College Park, Maryland 20742, USA.
Nature Communications 7, 11441 (2016)
Acknowledgement of Support:
This research used resources of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. X.Y., E.H., K.S., H.G., and X.-Q.Y. were supported by the U.S. DOE, the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies under Contract No. DE-SC00112704. The authors acknowledge the technical support by Dr. Wenqian Xu from Beamline 17-BM-B of Advanced Photon Source at Argonne National Laboratory. S.Z., H.Y. and C.B.M. acknowledge the support of MRSEC award No. DMR-1120901. S.Z. also acknowledges the support of the NatureNet Science Fellowship. Q.M. and Yimei Z. are supported by DOE/BES, Division of Materials Science and Engineering, under Contract No. DE-SC0012704. Those in-situ and analytical TEM experiments performed by J.L. and E.A.S. were supported as part of the Center for Mesoscale Transport Properties, an Energy Frontier Research Center supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, under award #DE-SC0012673. Yizhou Z. and Y.M. acknowledge the support of the Minta Martin award at University of Maryland, and the computational resources from Extreme Science and Engineering Discovery Environment (XSEDE) supported by National Science Foundation Grant No. TG-DMR130142 and from University of Maryland supercomputing resources.
2016-6626 | INT/EXT | Newsroom









