Watching Metals Oxidize at the Atomic Scale
November 16, 2023
 enlarge
enlarge
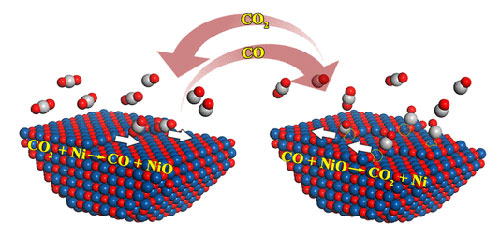
Simultaneously occurring forward reaction of NiO growth (left) and backward reaction of NiO reduction (right) were observed during Ni oxidation in CO2. This phenomenon had not been previously observed because of the difficulty in probing the fast dynamics of the local atomic configurations of the gas-solid interface under the reaction conditions of elevated temperature and pressure.
Scientific Achievement
A collaborative team of CFN users and staff used environmental transmission electron microscopy (TEM) to observe gas-solid reactions of a nickel (Ni) surface exposed to the oxidizing environment of carbon dioxide (CO2) — in real time at the atomic scale. The team discovered here-to-fore unexpected reaction dynamics, where oxidation and reduction occur at the same time, due to the countering effect of the gaseous carbon monoxide (CO) oxidation product.
Significance and Impact
Noncatalytic gas-solid surface reactions, like Ni oxidation, are industrially important. Atomistic insights into the rich dynamics, gleaned from in-situ TEM measurements, are useful for steering these types of reactions in desirable directions.
Research Details
Noncatalytic gas-solid reactions are a large group of heterogeneous reactions that are usually assumed to occur irreversibly because of the strong driving force to favor the forward direction toward the product formation. Using the example of Ni oxidation into NiO with CO2, this work demonstrates the existence of the reverse element that results in the NiO reduction from the countering effect of the gaseous product of CO. Using in-situ electron microscopy observations and atomistic modeling, the work shows that the oxidation process occurs via preferential CO2 adsorption along step edges that results in the step-flow growth of NiO layers, and the presence of Ni atoms on the flat NiO surface promotes the nucleation of NiO layers. Simultaneously, the NiO reduction happens via preferential step-edge adsorption of CO that leads to the receding motion of atomic steps, and the presence of Ni vacancies in the NiO surface facilitates the CO-adsorption-induced surface pitting. Based on the in-situ electron microscopy observations, temperature and CO2 pressure effect maps are constructed to illustrate the spatiotemporal dynamics of the competing NiO redox reactions. These results demonstrate the rich gas-solid surface reaction dynamics induced by the co-existing forward and reverse reaction elements. They suggest a practical applicability in manipulating gas-solid reactions via controlling the gas environment or atomic structure of the solid surface to steer the reaction toward the desired direction.
- In situ environmental TEM was used to atomically monitor Ni oxidation in CO2
- Ambient-pressure XPS and flow-reactor measurements cross-validated the in situ TEM observations
- The CFN Electron Microscopy and Theory & Computation Facilities were used for this work
Publication Reference
Chen X.B., Wang J.Y., Zhu Y.G., Xie Z., Ye S., Kisslinger K., Hwang S., Zakharov D., Zhou G.W. ”Atomistic Origins of Reversible Noncatalytic Gas-Solid Interfacial Reactions.” Journal of American Chemical Society 145, 7 (2023).
DOI: https://doi.org/10.1021/jacs.2c10083
OSTI: https://www.osti.gov/biblio/1969356
Acknowledgment of Support
This work was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award No. DE-SC0001135. This research used resources of the Center for Functional Nanomaterials and the Scientific Data and Computing Center, a component of the Computational Science Initiative, at Brookhaven National Laboratory, which is supported by the US Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-SC0012704.
2023-21660 | INT/EXT | Newsroom