Platinum-free Nanocatalysts Provide a Fuel Cell Boost
March 30, 2020
 enlarge
enlarge
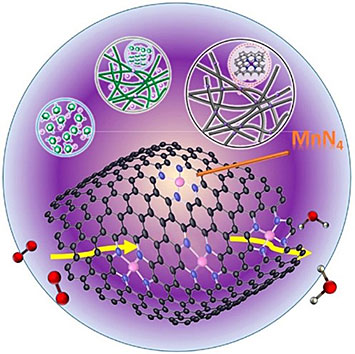
Schematic of Mn- and N- doped catalyst embedded in carbon for the oxygen reduction reaction in fuel cells. Calculations showed that the reaction occurs on MnN4 sites.
What is the scientific achievement?
A CFN user project investigated Mn- and N- doped catalysts (Mn-N-C) for the oxygen reduction reaction in fuel cells using combined computational and experimental methods. Calculations showed that the Mn-N-C catalyst has the potential to achieve a performance near that of a Pt catalyst (60 mV lower in terms of half-wave potential). Experiments showed the new catalyst has superior stability over 10,000 cycles compared to an Fe catalyst, degrading 75% percent less even after undergoing twice as many potential cycles.
Why does this achievement matter?
Polymer electrolyte membrane fuel cells have the potential to reduce energy use, pollutant emissions, and dependence on fossil fuels. Efficient, stable catalysts that are free from platinum group metals are key for widespread fuel-cell adoption.
What are the details?
The development of both platinum group metal-free and iron-free electrocatalysts is an important goal to achieve low-cost and long-term stability of polymer electrolyte membrane fuel cells. It has been suggested that manganese catalysts are a promising candidate for the oxygen reduction reaction (ORR). In this work, the first-principles density functional theory (DFT) calculations and micro-kinetics analysis predict the ORR kinetics of atomically dispersed MnN4 active sites in a carbon layer. Also, electrochemical tests and microscopic characterization were performed to demonstrate the structure of the Mn catalyst and its enhanced ORR performance. DFT calculations show ORR on MnN4 sites occurs via a four-electron pathway and a half-wave potential of MnN4 is only 60 mV lower than that of a Pt catalyst. Structural characterization exhibits the presence of atomically dispersed Mn active sites. Electrochemical measurements show the Mn catalysts can promote four-electron ORR with comparable catalytic activities of Fe catalysts in acids. Importantly, the Mn catalyst exhibits excellent potential cyclic stability – only losing 20 mV after 10,000 cycles, while Fe catalysts lose 80 mV after 5,000 cycles under the same conditions. This work provides the theoretical background along with experimental validation for the promising high-performance of this Mn catalyst for ORR in acidic medium.
CFN Capabilities
The CFN Electron Microscopy facility was used for high-resolution TEM and HAADF characterization.
Publication Reference
K. Liu, Z. Qiao, S. Hwang, Z. Liu, H. Zhang, D. Su, H. Xu, G. Wu, G. Wang, Mn- and N- doped carbon as promising catalysts for oxygen reduction reaction: Theoretical prediction and experimental validation, Applied Catalysis B: Environmental 243, 195 (2019).
DOI: https://doi.org/10.1016/j.apcatb.2018.10.034
Previous CFN Highlight: https://www.bnl.gov/cfn/research/highlights/news.php?a=216871
Acknowledgement of Support
This research was ?nancially supported by O?ce of Energy E?ciency and Renewable Energy, U.S. Department of Energy (Grant no. DE-EE0008075). G.F. Wang also acknowledges the research grants from National Science Foundation (Grant No. CBET-1804534 and CMMI-1662615). G. Wu also thanks the ?nancial support from National Science Foundation (CBET- 1604392). The authors gratefully acknowledge the computational resources provided by the University of Pittsburgh Center for Research Computing as well as the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation grant number ACI-1053575.
2020-17196 | INT/EXT | Newsroom









